Abstract
A gadolinium compound [Gd(TPPS)] n ·nH3O (1) (TPPS = tetra(4-sulfonatophenyl)porphyrin) was synthesized by a hydrothermal reaction. Compound 1 was characterized by FT-IR, UV–vis spectra, fluorescence, quantum yield, luminescence lifetime, CV and X-ray diffraction analyses. Compound 1 features a three-dimensional (3-D) porous open framework with lattice water molecules residing at the voids. The 3-D framework is comprised of TPPS moieties and Gd3+ nodes. Photoluminescence measurements reveal that compound 1 exhibits a sharp emission band in the red region with an emission quantum yield being of 5.8 %. Nanosecond transient spectra discover that the fluorescence lifetime of 1 is 11.7 ns. The voltammetric behavior of 1 displays one reduction and two oxidation potentials at −1.75, −0.71 and −1.07 V, respectively.
Graphical Abstract
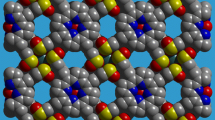
A gadolinium compound was synthesized via a hydrothermal reaction. It features a 3-D porous open framework which is comprised of TPPS moieties and Gd3+ nodes. It exhibits a sharp emission band in the red region with an emission quantum yield being of 5.8 % and a fluorescence lifetime being of 11.7 ns. The CV displays one reduction and two oxidation potentials.








Similar content being viewed by others
References
A. Fateeva, P.A. Chater, C.P. Ireland, A.A. Tahir, Y.Z. Khimyak, P.V. Wiper, J.R. Darwent, M.J. Rosseinsky, Angew. Chem. Int. Ed. 51, 7440 (2012)
A. Fateeva, S. Devautour-Vinot, N. Heymans, T. Devic, J.M. Grenèche, S. Wuttke, S. Miller, A. Lago, C. Serre, G.D. Weireld, G. Maurin, A. Vimont, G. Férey, Chem. Mater. 23, 4641 (2012)
X.S. Wang, M. Chrzanowski, C. Kim, W.Y. Gao, L. Wojtas, Y.S. Chen, X.P. Zhang, S. Ma, Chem. Commun. 48, 7173 (2012)
C. Zou, C.D. Wu, Dalton Trans. 41, 3879 (2012)
J.C. Chambron, V. Heitz, J.P. Sauvage, in The Porphyrin Handbook, ed. by K.M. Kadish, K.M. Smith, R. Guilard (Academic, New York, 2000), p. 6. (ch. 40)
K.M. Kadish, E. Van Caemelbecke, G. Royal, in The Porphyrin Handbook, ed. by K.M. Kadish, K.M. Smith, R. Guilard (Academic, San Diego, 2000), p. 8. (1)
C.M. Drain, A. Varotto, I. Radivojevic, Chem. Rev. 109, 1630 (2009)
J. Jiang, D.K.P. Ng, Acc. Chem. Res. 42, 79 (2009)
C. Bucher, C.H. Devillers, J.C. Moutet, G. Royal, E. Saint-Aman, Coord. Chem. Rev. 253, 21 (2009)
S. Matsunaga, N. Endo, W. Mori, Eur. J. Inorg. Chem. 29, 4550 (2011)
W. Morris, B. Volosskiy, S. Demir, F. Gándara, P.L. McGrier, H. Furukawa, D. Cascio, J.F. Stoddart, O.M. Yaghi, Inorg. Chem. 51, 6443 (2012)
N.C. Smythe, D.P. Butler, C.E. Moore, W.R. McGowan, A.L. Rheingold, L.G. Beauvais, Dalton Trans. 41, 7855 (2012)
C. Zou, M.H. Xie, G.Q. Kong, C.D. Wu, Cryst. Eng. Comm. 14, 4850 (2012)
J. Demel, P. Kubát, F. Millange, J. Marrot, I. Císařová, K. Lang, Inorg. Chem. 52, 2779 (2013)
W.T. Chen, Y. Yamada, G.N. Liu, A. Kubota, T. Ichikawa, Y. Kojima, G.C. Guo, S. Fukuzumi, Dalton Trans. 40, 12826 (2011)
Rigaku, CrystalClear Version 1.35 (Rigaku Corporation, Tokyo, 2002)
Siemens, SHELXTLTM Ver. 5 Reference Manual (Siemens Energy, Automation Inc, USA, 1994)
P. Yang, J.Z. Wu, Y. Yu, Inorg. Chim. Acta 362, 1907 (2009)
Y.J. Li, Q. Liang, H.H. Song, M.Y. Jia, S.K. Shi, J.J. Zhang, Wuji Huaxue Xuebao (Chinese J. Inorg. Chem.) 25, 725 (2009)
M. Gouterman, in The Porphyrins, vol. III, ed. by D. Dolphin (Academic, New York, 1978)
K.M. Kadish, E. Van Caemelbecke, F. D’Souza, M. Lin, D.J. Nurco, C.J. Medforth, T.P. Forsyth, B. Krattinger, K.M. Smith, S. Fukuzumi, I. Nakanishi, J.A. Shelnutt, Inorg. Chem. 38, 2188 (1999)
Acknowledgments
We greatly thank the financial support of the NSF of China (21361013), the NSF of Jiangxi Province (20132BAB203010, 20114BAB203028), the science and technology project of Jiangxi Provincial Department of Education (GJJ14554, GJJ13555) and the open foundation (No. 20130014) of the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WT., Hu, RH., Chen, HL. et al. Synthesis, characterization and properties of a gadolinium tetra(4-sulfonatophenyl)porphyrin. J IRAN CHEM SOC 12, 277–282 (2015). https://doi.org/10.1007/s13738-014-0482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0482-y



