Abstract
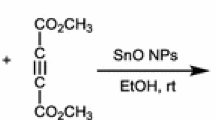
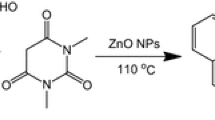
An efficient and high-yielding protocol using nano-ZnO (50–100 nm) as an efficient heterogeneous catalyst for one-pot three-component synthesis of pharmacologically significant furanone derivatives by the condensation of aromatic aldehyde, amine and dimethylacetylenedicarboxylate has been developed. Since the catalyst is heterogeneous, it can be easily separated and recycled several times without much loss of its catalytic activity. Use of aqueous medium, recyclable nanocatalyst, operational simplicity, non-chromatographic purification technique, excellent yield and short reaction time makes this approach an attractive protocol for the synthesis of 3,4,5-trisubstituted furan-2(5H)-ones.





Similar content being viewed by others
References
S. Miao, R.J. Andersen, J. Org. Chem. 56, 6275 (1991)
M. Kotora, E. Negishi, Synthesis 1, 121 (1997)
M. Pour, M. Spulak, V. Buchta, P. Kubanova, M. Voprsalova, V. Wsol, H. Fakova, P. Koudelka, H. Pourova, J. Med. Chem. 44, 2701 (2001)
E. Lattmann, N. Sattayasai, C.S. Schwalbe, S. Niamsanit, D.C. Billington, P. Lattmann, C.A. Langley, H. Singh, S. Dunn, Curr. Drug Discov. Technol. 3, 125 (2006)
V. Weber, P. Coudert, C. Rubat, E. Duroux, D. Vallee-Goyet, D. Gardette, M. Bria, E. Albuisson, F. Leal, J.C. Gramain, J. Couquelet, M. Madesclaire, Bioorg. Med. Chem. 10, 1647 (2002)
A. El-Tombary, Y. Abdel-Ghany, A. Belal, E.-D. Shams, F. Soliman, Med. Chem. Res. 20, 865 (2011)
E. Lattmann, W.O. Ayuko, D. Kinchinaton, C.A. Langley, H. Singh, L. Karimi, M.J. Tisdale, J. Pharm. Pharmacol. 55, 1259 (2003)
R.T. LaLonde, L. Bu, A. Henwood, J. Fiumano, L. Zhang, Chem. Res. Toxicol. 10, 1427 (1997)
G.Y. Liu, B.Q. Guo, W.N. Chen, C. Cheng, Q.L. Zhang, M.B. Dai, J.R. Sun, P.H. Sun, W.M. Chen, Chem. Biol. Drug Des. 79, 628 (2012)
C. Kalinski, H. Lemoine, J. Schmidt, C. Burdack, J. Kolb, M. Umkehrer, G. Ross, Synthesis 24, 4007 (2008)
H. Mecadon, M.R. Rohman, I. Kharbangar, B.M. Laloo, I. Kharkongor, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 3228 (2011)
K.S. Leschkies, R. Divakar, J. Basu, E.E. Pommer, J.E. Boercker, C.B. Carter, U.R. Kortshagen, D.J. Norris, E.S. Aydil, Nano Lett. 7, 1793 (2007)
L. Liao, H.B. Lu, J.C. Li, H. He, D.F. Wang, D.J. Fu, C. Liu, J. Phys. Chem. C 111, 1900 (2007)
Y.N. Xia, P.D. Yang, Y.G. Sun, Y.Y. Wu, B. Mayers, B. Gates, Y.D. Yin, F. Kim, H.Q. Yan, Adv. Mater. 15, 353 (2003)
N.M. Franklin, N.J. Rogers, S.C. Apte, G.E. Batley, G.E. Gadd, P.S. Casey, Environ. Sci. Tech. 41, 8484 (2007)
I. M. David, D. Minoo, S. Peyman, T. Laleh, Arkivoc xi (2011) 156
F.M. Moghaddam, H. Saeidian, Z. Mirjafary, A. Sadeghi, J. Iran. Chem. Soc. 6, 317–324 (2009)
R. Hekmatshoa, G.N. Kenary, S. Sadjadi, Y.S. Beheshtiha, Syn. Comm. 40, 2007 (2010)
I. Yavari, S. Beheshtiran, J. Iran. Chem. Soc. 8, 1030 (2011)
N.B. Carter, R. Mabon, A.M.E. Richecœur, J.B. Sweeney, Tetrahedron 58, 9117 (2002)
M. Bassetti, A. D’Annibale, A. Fanfoni, F. Minissi, Org. Lett. 7, 1805 (2005)
Y. Fukuta, I., Matsuda, K. Itoh, Tetrahedron Lett. 42 (2001) 1301
M. Bella, G. Piancatelli, A. Squarcia, Tetrahedron 57, 4429 (2001)
F. Bellina, M. Biagetti, A. Carpita, R. Rossi, Tetrahedron 57, 2857 (2001)
Y. Kim, N.-H. Nam, Y.-J. You, B.-Z. Ahn, Bioorg. Med. Chem. Lett. 12, 719 (2002)
A. Kumar, B. Ahmed, B. Srivastawa, Vaishali. Der Pharma Chem 4, 383 (2012)
S.N. Murthy, B. Madhav, A.V. Kumar, K.R. Rao, Y.V.D. Nageswar, Tetrahedron 65, 5251 (2009)
L. Nagarapu, U.N. Kumar, P. Upendra, R. Bantu, Syn. Comm. 42, 2139 (2012)
Acknowledgments
The authors are thankful to the Principal, Deogiri College Aurangabad, for providing laboratory facilities and constant encouragement during the work. We thank the Department of Physics, Pune University, for providing the Sophisticated Analytical and Instrument (SAIF) Facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tekale, S.U., Kauthale, S.S., Pagore, V.P. et al. ZnO nanoparticle-catalyzed efficient one-pot three-component synthesis of 3,4,5-trisubstituted furan-2(5H)-ones. J IRAN CHEM SOC 10, 1271–1277 (2013). https://doi.org/10.1007/s13738-013-0266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0266-9