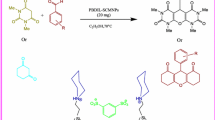
Abstract
High yielding preparation of structurally different β-hydroxy 1,4-disubstituted 1,2,3-triazole from the regio-selective reaction of epoxides 2a–2c with wide range of terminal alkynes 1a–1j, and sodium azide in the presence of catalytic amount, complexed form of homogeneous catalyst [bis 1,4-mono ester hydroxy anthraquinone copper(II)] (5.0 mol%), [AQ2Cu(II)] 7 at 25 °C in water has been described. To benefit from the recovery and reuse of the catalyst, a novel heterogeneous nanoparticles of catalyst [bis 1,4-mono ester hydroxy anthraquinone copper(II) aminopropyl silica gel] (5.0 mol%), [AQ2Cu(II)-APSiO2] 13 bearing oxygen anthraquinone ligands with strong copper(II) affinity was easily prepared by using silica gel as a suitable support. The heterogeneous catalyst was fully characterized by XRD, SEM, AFM, ICP, TG methods for analysis of nitrogen adsorption, and FT-IR techniques. The remarkable features of this protocol are high yields, short reaction times, a cleaner reaction profile in an environmentally benign solvent (H2O), one-pot procedure and the method is applicable to large-scale operation without any problem. Furthermore, the catalyst could be recovered and easily removed by simple filtration of the reaction mixture and it was recycled ten times showing negligible copper leaching. In their uncomplexed form of homogeneous catalyst [AQ2Cu(II)] 7 and heterogeneous catalyst [AQ2Cu(II)-APSiO2] 13, the anthraquinone ligand 6 and [AQ-APSiO2] 12 are very efficient copper scavengers able to catalyze the 1,3-dipolar cycloaddition reaction of azides with alkynes (CuAAC) without pre-synthesis of catalysts.
Graphical Abstract













Similar content being viewed by others
References
B.S. Holla, M. Mahalinga, M.S. Karthikeyan, B. Poojary, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem. 40, 1173 (2005)
K. Dabak, O. Sezer, A. Akar, O. Anac, Eur. J. Med. Chem. 38, 215 (2003)
W.S. Horne, M.K. Yadav, C.D. Stout, M.R. Ghadiri, J. Am. Chem. Soc. 126, 15366 (2004)
A.D. Moorhouse, A.M. Santos, M. Gunaratnam, M. Moore, S. Neidle, J.E. Moses, J. Am. Chem. Soc. 128, 15972 (2006)
A.D. Moorhouse, J.E. Moses, Chem. Med. Chem. 3, 715 (2008)
C. Mamat, T. Ramenda, F.R. Wuest, Mini-Rev. Org. Chem. 6, 21 (2009)
M.D. Best, Biochemistry 48, 6571 (2009)
M. Whiting, J.C. Tripp, Y.C. Lin, W. Lindstrom, A.J. Olson, J.H. Elder, K.B. Sharpless, V.V. Fokin, J. Med. Chem. 49, 7697 (2006)
M.J. Giffin, H. Heaslet, A. Brik, Y.-C. Lin, G. Cauvi, C.-H. Wong, D.E. McRee, J.H. Elder, C.D. Stout, B.E. Torbett, J. Med. Chem. 51, 6263 (2008)
S.H. Kim, H.S. Choi, J. Kim, S.J. Lee, D.T. Quang, J.S. Kim, Org. Lett. 12, 560 (2010)
L.-F. Lutz, Angew. Chem. Int. Ed. 46, 1018 (2007)
J. Zhan, D. Tian, H. Li, New J. Chem. 33, 725 (2009)
S.Y. Park, J.H. Yoon, C.S. Hong, R. Souane, J.S. Kim, S.E. Matthews, J. Vicens, J. Org. Chem. 73, 8212 (2008)
K. Chang, L. Su, A. Senthilvelan, W. Chung, Org. Lett. 9, 3363 (2007)
V. Haridas, K. Lal, Y.K. Sharma, S. Upreti, Org. Lett. 10, 1645 (2008)
P. Wu, M. Malkoch, J.N. Hunt, R. Vestberg, E. Kaltgrad, M.G. Finn, V.V. Fokin, K.B. Sharpless, C.J. Hawker, Chem. Commun. 48, 5775 (2005)
G. Franc, A.K. Kakkar, Chem. Eur. J. 15, 5630 (2009)
R. Huisgen, Angew. Chem. Int. Ed. Engl. 2, 565 (1963)
K.V. Gothelf, K.A. Jorgensen, Chem. Rev. 98, 863 (1998) (see the references cited therein)
R. Huisgen, 1,3-Dipolar cycloaddition chemistry, chap 1 A. Padwa (ed.) (Wiley, New York, 1984) p. 1
M. Liu, O. Reiser, 1,3-Dipolar cycloaddition chemistry, Org. Lett. 13, 1102 (2011) (see the references cited therein)
C. Spiteri, J.E. Moses, Angew. Chem. Int. Ed. 49, 31 (2010)
M. Fuchs, W. Goessler, C. Pilger, C.O. Kappe, Adv. Synth. Catal. 352, 323 (2010)
G.-C. Kuang, H.A. Michaels, J.T. Simmons, R.J. Clark, L. Zhu, J. Org. Chem. 75, 6540 (2010)
A.E. Cohrt, J.F. Jensen, T.E. Nielsen, Org. Lett. 12, 5414 (2010)
J. T. Fletcher, M. E. Keeney, S. E. Walz, Synthesis 19, 3339 (2010)
M. Xu, C. Kuang, Z. Wang, Q. Yang, Y. Jiang. Synthesis 2, 223 (2011)
S. Chandrasekhar, M. Seenaiah, A. Kumar, C.R. Reddy, S.K. Mamidyal, C.G. Kumar, S. Balasubramanian, Tetrahedron Lett. 52, 806 (2011)
K.D. Hänni, D.A. Leigh, Chem. Soc. Rev. 39, 1240 (2010)
J.M. Holub, K. Kirshenbaum, Chem. Soc. Rev. 39, 1325 (2010)
F. Santoyo-Gonzalez, F. Hernandez-Mateo, Chem. Soc. Rev. 38, 3449 (2009)
R.A. Decre’au, J.P. Collman, A. Hosseini, Chem. Soc. Rev. 39, 1291 (2010)
P. Appukkuttan, V.P. Mehtaa, E. Van der Eycken, Chem. Soc. Rev. 39, 1467 (2010)
D. Wang, Na. Li, M. Zhao, W. Shi, C. Ma, B. Chen, Green Chem. 12, 2120 (2010) (see references therein)
C.Le. Droumaguet, C. Wang, Q. Wang, Chem. Soc. Rev. 39, 1233 (2010)
C.O. Kappe, E. Van der Eycken, Chem. Soc. Rev. 39, 1280 (2010)
A.H. El-Sagheer, T. Brown, Chem. Soc. Rev. 39, 1388 (2010)
H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 40, 2004 (2001)
M.G. Finn, V.V. Fokin, Chem. Soc. Rev. 39, 1231 (2010)
S.K. Mamidyala, M.G. Finn, Chem. Soc. Rev. 39, 1252 (2010)
C.E. Hoyle, A.B. Lowe, C.N. Bowman, Chem. Soc. Rev. 39, 1355 (2010)
D. Urankar, M. Steinbucher, J. Kosjek, J. Kosmrlj, Tetrahedron 66, 2602 (2010)
H. Elamari, F. Meganem, J. Herscovici, C. Girard, Tetrahedron Lett. 52, 658 (2011)
T. Nakamura, T. Terashima, K. Ogata, S.I. Fukuzawa, Org. Lett. 13, 620 (2011)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596 (2002)
K.B. Sharpless, V.V. Fokin, L.G. Green, V.V. Rostovtsev, Angew. Chem. Int. Ed. 114, 2708 (2002)
C.W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 67, 3057 (2002)
M. Meldal, C.W. Tornøe, Chem. Rev. 108, 2952 (2008) (see the references cited therein)
J.E. Hein, V.V. Fokin, Chem. Soc. Rev. 39, 1302 (2010) (see the references cited therein)
W.S. Brotherton, H.A. Michaels, J.T. Simmons, R.J. Clark, N.S. Dalal, L. Zhu, Org. Lett. 11, 4954 (2009)
H. Sharghi, M. H. Beyzavi, A. Safavi, M. M. Doroodmand, R. Khalifeh, Adv. Synth. Catal. 351, 2391 (2009) (see the references cited therein)
L. Durán Pachón, J.H. Van Maarseveen, G. Rothenberg, Adv. Synth. Catal. 347, 811 (2005)
T. Miao, L. Wang, Synthesis 3, 363 (2008)
A. Coelho, P. Diz, O. Caamano, E. Sotelo, Adv. Synth. Catal. 352, 179 (2010)
S. Chassing, M. Kumarraja, A.S.S. Sido, P. Pale, J. Sommer, Org. Lett. 9, 883 (2007)
S. Chassaing, A.S.S. Sido, A. Alix, M. Kumarraja, P. Pale, J. Sommer, Chem. Eur. J. 14, 6713 (2008)
P. Kuhn, A. Alix, M. Kumarraja, B. Louis, P. Pale, J. Sommer. Eur. J. Org. Chem. 3, 423 (2009)
V. Bénéteau, A. Olmos, T. Boningari, J. Sommer, P. Pale, Tetrahedron Lett. 51, 3673 (2010)
K. Yamaguchi, T. Oishi, T. Katayama, N. Mizuno, Chem. Eur. J. 15, 10464 (2009)
M.L. Kantam, V.S. Jaya, B. Sreedhar, M.M. Rao, B.M. Choudary, J. Mol. Catal. A. 256, 273 (2006)
T. Katayama, K. Kamata, K. Yamaguchi, N. Mizuno, ChemSusChem 2, 59 (2009)
C. Girard, E. Onen, M. Aufort, S. Beauviere, E. Samson, J. Herscovici, Org. Lett. 8, 1689 (2006)
B. Lipshutz, B.R. Taft, Angew. Chem. Int. Ed. 45, 8235 (2006)
C.-T. Lee, S. Huang, B.H. Lipshutz, Adv. Synth. Catal. 351, 3139 (2009)
F. Alonso, Y. Moglie, G. Radivoy, M. Yus, Adv. Synth. Catal. 352, 3208 (2010)
F. Alonso, Y. Moglie, G. Radivoy, M. Yus, Tetrahedron Lett. 50, 2358 (2009)
M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard, F. Taran, Angew. Chem. Int. Ed. 121, 6030 (2009)
A. Megia-Fernandez, M. Ortega-Muñoz, J. Lopez-Jaramillo, F. Hernandez-Mateo, F. Santoyo-Gonzalez, Adv. Synth. Catal. 352, 3306 (2010)
B.R. Buckley, S.E. Dann, H. Heaney, E.C. Stubbs, Eur. J. Org. Chem. 4, 770 (2011)
L.A. Bigelow, H.H. Reynolds, Org. Synth. 6, 476 (1941)
J.W. Lown, Anthracycline and anthracenedione-based anticancer agents, chap IV. (Elsevier, 1988), pp. 129–161 (see references therein)
T. Moriuchi, T. Watanabe, I. Ikeda, A. Ogawa, T. Hirao, Eur. J. Inorg. Chem. 1, 277 (2001)
A. Quach, V. Escax, L. Nicole, P. Goldner, O. Guillot-Noël, P. Aschehoug, P. Hesemann, J. Moreau, D. Gourier, C. Sanchez, J. Mater. Chem. 17, 2552 (2007)
M. Shamsipur, A. Besharati-Seidani, J. Fasihi, H. Sharghi, Talanta 83, 674 (2010)
M. Barzegar, M.F. Mousavi, H. Khajesharifi, M. Shamsipur, H. Sharghi, IEEE Sens. J. 5, 392 (2005)
A. Rahmani, M.F. Mousavi, S.M. Golabi, M. Shamsipur, H. Sharghi, Chem. Anal. 49, 359 (2004)
S. Riahi, M.F. Mousavi, M. Shamsipour, H. Sharghi, Electroanalysis 15, 1561 (2003)
M. Shamsipur, A. Avanes, M. Javanbakht, M.R. Ganjali, H. Sharghi, Anal. Sci. 18, 875 (2002)
M. Shamsipur, F. Raoufi, H. Sharghi, Talanta 52, 637 (2000)
H. Sharghi, M.H. Beyzavi, M.M. Doroodmand, Eur. J. Org. Chem. 24, 4126 (2008)
H. Sharghi, M. Aberi, M.M. Doroodmand, Adv. Synth. Catal. 350, 2380 (2008)
H. Sharghi, A.R. Salimi-Beni, Synthesis 1, 2900 (2004)
H. Sharghi, O. Asemani, R. Khalifeh, Synth. Commun. 38, 1128 (2008)
H. Sharghi, M. Hosseini-Sarvari, F. Moeini, Can. J. Chem. 86, 1044 (2008)
H. Sharghi, M. Jokar, Heterocycles 71, 2721 (2007)
H. Sharghi, A.R. Salimi-Beni, R. Khalifeh, Helv. Chim. Acta 90, 1373 (2007)
H. Sharghi, A.R. Salimi-Beni, ARKIVOC xiii, 1 (2007)
A. Zare, A. Hasaninejad, M.H. Beyzavi, A. Parhami, A.R. Zare, M.A. Khalafi-Nezhad, H. Sharghi, Can. J. Chem. 86, 317 (2008)
H. Sharghi, R. Khalifeh, Can. J. Chem. 86, 426 (2008)
H. Sharghi, M. Jokar, M.M. Doroodmand, R. Khalifeh, Adv. Synth. Catal. 352, 3031 (2010)
H. Sharghi, M. Jokar, M.M. Doroodmand, Adv. Synth. Catal. 353, 426 (2011)
H. Sharghi, R. Khalifeh, Heterocycles 71, 1601 (2007)
H. Firouzabadi, N. Iranpoor, A. Khoshnood, J. Mol. Catal. A 274, 109 (2007)
A. Khalafi-Nezhad, M. N. Soltani Rad, A. Khoshnood. Synthesis 16, 2552 (2003)
A. Khalafi-Nezhad, M. N. Soltani Rad, A. Khoshnood. Synthesis 4, 583 (2004)
A. Brik, J. Alexandratos, Y.-C. Lin, J.H. Elder, A.J. Olson, A. Wlodawer, D.S. Goodsell, C.H. Wong, Chem. Biol. Chem. 6, 1167 (2005)
K.R. Reddy, C.U. Maheswari, K. Rajgopal, M.L. Kantam, Synth. Commun. 38, 2158 (2008)
H. Sharghi, M. Hosseini-Sarvari, F. Moeini, R. Khalifeh, A.R. Salimi-Beni, Helv. Chim. Acta. 93, 435 (2010)
H. Firouzabadi, N. Iranpoor, A. Garzan, Adv. Synth. Catal. 347, 1925 (2005)
H. Firouzabadi, N. Iranpoor, M. Abbasi, Adv. Synth. Catal. 351, 755 (2009)
H. Firouzabadi, N. Iranpoor, A.A. Jafari, E. Riazymontazer, Adv. Synth. Catal. 348, 434 (2006)
H. Firouzabadi, N. Iranpoor, A. Khoshnood, Catal. Commun. 9, 529 (2008)
F. Cozzi, Adv. Synth. Catal. 348, 1367 (2006) (see the references cited therein)
K. Binnemans, Chem. Rev. 109, 4283 (2009)
C. Baleizão, H. Garcia, Chem. Rev. 106, 3987 (2006) (see the references cited therein)
A.F. Trindade, P.M.P. Gois, C.A.M. Afonso, Chem. Rev. 109, 418 (2009) (see the references cited therein)
J.M. Fraile, J.I. García, J.A. Mayoral, Chem. Rev. 109, 360 (2009)
A.P. Wight, M.E. Davis, Chem. Rev. 102, 3589 (2002)
L. Yin, J. Liebscher, Chem. Rev. 107, 133 (2007)
A. Corma, H. Garcia, Adv. Synth. Catal. 348, 1391 (2006) (see the references cited therein)
S. Minakata, M. Komatsu, Chem. Rev. 109, 711 (2009) (see the references cited therein)
C.A. McNamara, M.J. Dixon, M. Bradley. Chem. Rev. 102, 3275 (2002) (see the references cited therein)
H. Bloom, L.H. Briggs, B. Cleverley, J. Chem. Soc. 178, 178 (1959)
H. Sharghi, R. Khalifeh, M.M. Doroodmand, Adv. Synth. Catal. 351, 207 (2009)
J.W. Daly, W.L. Padgett, M.T. Shamim, J. Med. Chem. 29, 1305 (1986)
A. Casaschi, R. Grigg, J.M. Sansano, Tetrahedron 56, 7553 (2000)
M. Mabrour, K. Bougrin, R. Benhida, A. Loupyc, M. Soufiaouia, Tetrahedron Lett. 48, 443 (2007)
Acknowledgments
We appreciate the funding from Shiraz University research council for the financial support in work. We are grateful to Dr. M. Nakoei and Mr. M. Sorouri for their helps and comments during the progress of this work. We are also thankful to Mr. M.S. Darvish Tafvizi and Mr. H. Sajedian Fard for running the NMR and mass spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharghi, H., Khoshnood, A., Doroodmand, M.M. et al. 1,4-Dihydroxyanthraquinone-copper(II) nanoparticles immobilized on silica gel: a highly efficient, copper scavenger and recyclable heterogeneous nanocatalyst for a click approach to the three-component synthesis of 1,2,3-triazole derivatives in water. J IRAN CHEM SOC 9, 231–250 (2012). https://doi.org/10.1007/s13738-011-0046-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-011-0046-3