Abstract
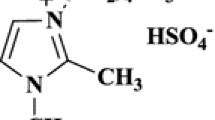
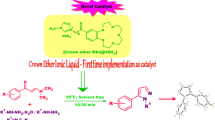
A facile and convenient protocol was developed for the synthesis of 5-arylidene-2-imino-4-thiazolidinones using solid basic catalyst immobilized onto supported ionic liquid-like phase (SILLP) in high yields (80–95%). The X-ray analysis of the representative compound established the Z configuration of the product at the chiral axis.


Similar content being viewed by others
References
A. Verma, S.K. Saraf, Eur. J. Med. Chem. 43, 897 (2008)
S. Singh, S.S. Parmar, K. Raman, V.I. Stenberg, Chem. Rev. 81, 175 (1981)
Z. Liu, Y. Huang, W. Zhang, L. Ma, J. Li, X. Wang, J. Li, J. Shen, J. Comb. Chem. 10, 632 (2008)
R. Ottanà, R. Maccari, M.L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. Di Paola, L. Sautebin, S. Cuzzocread, M.G. Vigorita, Bioorg. Med. Chem. 13, 4243 (2005)
R. Ottanà, R. Maccari, R. Ciurleo, P. Paoli, M. Jacomelli, G. Manao, G. Camici, C. Laggner, T. Langer, Bioorg. Med. Chem. 17, 1928 (2009)
T. Kato, T. Ozaki, K. Tamura, Y. Suzuki, M. Akima, N. Ohi, J. Med. Chem. 41, 4309 (1998)
T. Kato, T. Ozaki, K. Tamura, Y. Suzuki, M. Akima, N. Ohi, J. Med. Chem. 42, 3134 (1999)
M.V. Diurno, O. Mazzoni, E. Piscopo, A. Calignano, F. Giordano, A. Bolognesell, J. Med. Chem. 35, 2910 (1992)
A. Geronikaki, P. Eleftheriou, P. Vicini, I. Alam, A. Dixit, A.K. Saxena, J. Med. Chem. 51, 5221 (2008)
P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti, F. Zani, Bioorg. Med. Chem. 14, 3859 (2006)
R. Ottanà, S. Carotti, R. Maccari, I. Landini, G. Chiricosta, B. Caciagli, M.G. Vigorita, E. Mini, Bioorg. Med. Chem. Lett. 15, 3930 (2005)
R. Ottanà, R. Maccari, M.L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. Di Paola, L. Sautebin, S. Cuzzocrea, M.G. Vigorita, Bioorg. Med. Chem. 13, 4243 (2005)
P.C. Lv, C.F. Zhou, J. Chen, P.G. Liu, K.R. Wang, W.J. Mao, H.Q. Li, Y. Yang, J. Xiong, H.L. Zhu, Bioorg. Med. Chem. Lett. 19, 2819 (2009)
R.K. Rawal, R. Tripathi, S.B. Katti, C. Pannecouque, E.D. Clercq, Bioorg. Med. Chem. 15, 1725 (2007)
K.S. Ahmad, M. Yusuf, Eur. J. Med. Chem. 44, 2597 (2009)
X. Zhang, X. Li, D. Li, G. Qu, J. Wang, P.M. Loiseau, X. Fan, Bioorg. Med. Chem. Lett. 15, 6280 (2009)
T. Kline, K.C. Barry, S.R. Jackson, H.B. Felise, H.V. Nguyen, S.I. Miller, Bioorg. Med. Chem. Lett. 19, 1340 (2009)
M.C. Munson, A.W. Cook, J.A. Josey, C. Rao, Tetrahedron Lett. 39, 7223 (1998)
C.T. Sadashiva, J.N. Narendra Sharath Chandra, C.V. Kavitha, A. Thimmegowda, M.N. Subhash, K.S. Rangappa, Eur. J. Med. Chem. 44, 4848 (2009)
M.H. Shih, F. Ying Ke, Bioorg. Med. Chem. 12, 4633 (2004)
H. Zhou, Z. Wu, S. Zhai, A. Liu, A. Sun, R. Li, Y. Zhang, S. Ekins, P.W. Swaan, B. Fang, B. Zhang, B. Yan, J. Med. Chem. 51, 242 (2008)
B.L. Barreca, J. Balzarini, A. Chimirri, E.D. Clercq, L.D. Luca, H.D. Höltje, M. Höltje, A.M. Monforte, P. Monforte, C. Pannecouque, A. Rao, M. Zappalà, J. Med. Chem. 45, 5410 (2002)
J.A. Linthorst, Found. Chem. 12, 55 (2010)
M.T. Garcia, N. Gathergood, P.J. Scammells, Green Chem. 7, 9 (2007)
R. Franzen, Comb. Chem. 2, 195 (2000)
M.H. Valkenberg, C. deCastro, W.F. Hölderich, Green Chem. 4, 88 (2002)
E. Benazzi, A. Hirschauer, J.F. Joly, H. Olivier, J.Y. Berhard, Eur. Patent EP 0,553,009 28, 7 (1993)
C.P. Mehnert, Chem. Eur. J. 11, 50 (2004)
A. Riisager, B. Jørgensen, P. Wasserscheid, R. Fehrmann, Chem. Commun. 994 (2006)
R. Sugimura, K. Qiao, D. Tomida, C. Yokoyama, Catal. Commun. 8, 770 (2007)
J.Q. Wang, X.D. Yue, F. Cai, L.N. He, Catal. Commun. 8, 167 (2007)
M.J. Jin, A. Taher, H.J. Kang, M. Choi, R. Ryoo, Green Chem. 11, 309 (2009)
P.R. Likhar, S. Roy, M. Roy, M.S. Subhas, M. Lakshmi Kantam, Catal. Commun. 10, 728 (2009)
M.I. Burguete, H. Erytropel, E.G. Verdugo, S.V. Luis, V. Sans, Green Chem. 10, 401 (2008)
M. Mamaghani, A. Loghmanifar, M.R. Taati, Ultrason. Sonochem. 18, 45 (2011)
M. Nikpassand, M. Mamaghani, F. Shirini, K. Tabatabaeian, Ultrason. Sonochem. 17, 301 (2010)
M. Mamaghani, N.O. Mahmoodi, S. Fallah Ghassemi, J. Iran. Chem. Soc. 7, 972 (2010)
M. Mamaghani, S. Dastmard, Arkivoc 168 (2009)
H.A. Samimi, M. Mamaghani, K. Tabatabaeian, Heterocycles 75, 2825 (2008)
H.A. Samimi, M. Mamaghani, K. Tabatabaeian, J. Heterocycl. Chem. 45, 1765 (2008)
K. Tabatabaeian, M. Mamaghani, N. Mahmoodi, A. Khorshidi, J. Mol. Catal. A 270, 112 (2007)
Acknowledgments
The authors are grateful to the Research Council of University of Guilan for the financial support of this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saffari Jourshari, M., Mamaghani, M., Tabatabaeian, K. et al. A convenient synthesis of novel 5-arylidene-2-imino-4-thiazolidinones using base supported ionic liquid-like phase (SILLP) as efficient green catalyst. J IRAN CHEM SOC 9, 75–80 (2012). https://doi.org/10.1007/s13738-011-0012-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-011-0012-0