Abstract
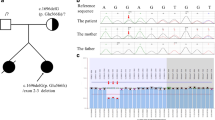
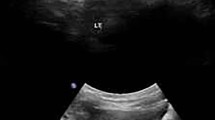
Identification of a monogenic etiology is possible in a proportion of patients with childhood-onset nephrolithiasis or nephrocalcinosis. Bartter syndrome (BS), a hereditary tubulopathy characterized by polyuria, hypokalemic alkalosis and growth retardation that rarely presents with isolated nephrocalcinosis. Patients with defect in renal outer medullary potassium channel, encoded by the KCNJ1 gene causing BS type 2, typically present during the neonatal period. We describe a 14-year-old girl with mild late-onset BS type 2 with reported pathogenic compound heterozygous variations in exon 2 of KCNJ1 (c.146G > A and c.657C > G). This patient presented with isolated medullary nephrocalcinosis due to hypercalciuria; absence of hypokalemia and metabolic alkalosis was unique. This case highlights the importance of screening the KCNJ1 gene in patients with hypercalciuria and nephrocalcinosis, even in older children.


Similar content being viewed by others
References
Braun DA, Lawson JA, Gee HY, Halbritter J, Shril S, Tan W, et al. Prevalence of monogenic causes in pediatric patients with nephrolithiasis or nephrocalcinosis. Clin J Am Soc Nephrol. 2016;11(4):664–72.
Daga A, Majmundar AJ, Braun DA, Gee HY, Lawson JA, Shril S, et al. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018;93(1):204–13.
Halbritter J, Baum M, Hynes AM, Rice SJ, Thwaites DT, Gucev ZS, et al. Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol. 2015;26(3):543–51.
Seyberth HW, Weber S, Komhoff M. Bartter's and Gitelman's syndrome. Curr Opin Pediatr. 2017;29(2):179–86.
Besouw MTP, Kleta R, Bockenhauer D. Bartter and Gitelman syndromes: questions of class. Pediatr Nephrol. 2019. https://doi.org/10.1007/s00467-019-04371-y.
Laghmani K, Beck BB, Yang SS, Seaayfan E, Wenzel A, Reusch B, et al. Polyhydramnios, transient antenatal Bartter's syndrome, and MAGED2 mutations. N Engl J Med. 2016;374(19):1853–63.
Seyberth HW. An improved terminology and classification of Bartter-like syndromes. Nat Clin Pract Nephrol. 2008;4(10):560–7.
Seys E, Andrini O, Keck M, Mansour-Hendili L, Courand PY, Simian C, et al. Clinical and genetic spectrum of Bartter syndrome type 3. J Am Soc Nephrol. 2017;28(8):2540–52.
Amar A, Majmundar AJ, Ullah I, Afzal A, Braun DA, Shril S, et al. Gene panel sequencing identifies a likely monogenic cause in 7% of 235 Pakistani families with nephrolithiasis. Hum Genet. 2019;138(3):211–9.
Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, et al. pH gating of ROMK (K(ir)1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci U S A. 1999;96(26):15298–303.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Finer G, Shalev H, Birk OS, Galron D, Jeck N, Sinai-Treiman L, et al. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr. 2003;142(3):318–23.
Landau D, Gurevich E, Sinai-Treiman L, Shalev H. Accentuated hyperparathyroidism in type II Bartter syndrome. Pediatr Nephrol. 2016;31(7):1085–90.
Fretzayas A, Gole E, Attilakos A, Daskalaki A, Nicolaidou P, Papadopoulou A. Expanding the spectrum of genetic mutations in antenatal Bartter syndrome type II. Pediatr Int. 2013;55(3):371–3.
Derst C, Konrad M, Kockerling A, Karolyi L, Deschenes G, Daut J, et al. Mutations in the ROMK gene in antenatal Bartter syndrome are associated with impaired K+ channel function. Biochem Biophys Res Commun. 1997;230(3):641–5.
Brochard K, Boyer O, Blanchard A, Loirat C, Niaudet P, Macher MA, et al. Phenotype–genotype correlation in antenatal and neonatal variants of Bartter syndrome. Nephrol Dial Transpl. 2009;24(5):1455–64.
Peters M, Jeck N, Reinalter S, Leonhardt A, Tonshoff B, Klaus GG, et al. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112(3):183–90.
Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13(2):183–8.
Walsh PR, Tse Y, Ashton E, Iancu D, Jenkins L, Bienias M, et al. Clinical and diagnostic features of Bartter and Gitelman syndromes. Clin Kidney J. 2018;11(3):302–9.
Bettinelli A, Ciarmatori S, Cesareo L, Tedeschi S, Ruffa G, Appiani AC, et al. Phenotypic variability in Bartter syndrome type I. Pediatr Nephrol. 2000;14(10–11):940–5.
Madrigal G, Saborio P, Mora F, Rincon G, Guay-Woodford LM. Bartter syndrome in Costa Rica: a description of 20 cases. Pediatr Nephrol. 1997;11(3):296–301.
Kurtz CL, Karolyi L, Seyberth HW, Koch MC, Vargas R, Feldmann D, et al. A common NKCC2 mutation in Costa Rican Bartter's syndrome patients: evidence for a founder effect. J Am Soc Nephrol. 1997;8(11):1706–11.
Li J, Hu S, Nie Y, Wang R, Tan M, Li H, et al. A novel compound heterozygous KCNJ1 gene mutation presenting as late-onset Bartter syndrome: case report. Medicine (Baltimore). 2019;98(34):e16738.
Gollasch B, Anistan YM, Canaan-Kuhl S, Gollasch M. Late-onset Bartter syndrome type II. Clin Kidney J. 2017;10(5):594–9.
Sharma A, Linshaw MA. A novel compound heterozygous ROMK mutation presenting as late onset Bartter syndrome associated with nephrocalcinosis and elevated 1,25(OH)(2) vitamin D levels. Clin Exp Nephrol. 2011;15(4):572–6.
Huang L, Luiken GP, van Riemsdijk IC, Petrij F, Zandbergen AA, Dees A. Nephrocalcinosis as adult presentation of Bartter syndrome type II. Neth J Med. 2014;72(2):91–3.
Jeck N, Derst C, Wischmeyer E, Ott H, Weber S, Rudin C, et al. Functional heterogeneity of ROMK mutations linked to hyperprostaglandin E syndrome. Kidney Int. 2001;59(5):1803–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This article does not contain any interventional studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the parents of the patients included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Khandelwal, P., Sabanadesan, J., Sinha, A. et al. Isolated nephrocalcinosis due to compound heterozygous mutations in renal outer medullary potassium channel. CEN Case Rep 9, 232–236 (2020). https://doi.org/10.1007/s13730-020-00464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00464-y