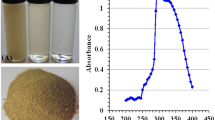
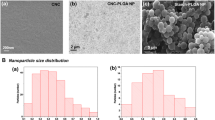
Abstract
In recent years, there has been a growing emphasis on eco-friendly methods to protect plants from pathogens, aiming to enhance crop yields while minimizing pesticide use. In this context, we synthesized salicylic acid-encapsulated chitosan nanoparticles (SA-CNPs) and evaluated their effectiveness in safeguarding tomato plants against Fusarium wilt caused by Fusarium oxysporum f. sp. Lycopersici (FOL). SA-CNPs at concentrations of 0.01%, 0.05%, 0.1%, 0.15%, and 0.2% w/v were prepared using ionic gelation and characterized through scanning electron microscopy, zeta potential, X-ray diffraction, and Fourier transform infrared spectroscopy techniques. The results revealed an average particle size ranging from 30 to 300 nm, with zeta potential values − 30 to − 53 mV, confirming exceptional stability. Encapsulation efficiency varied from 19 to 90%. In antifungal tests, 0.2% SA-CNPs exhibited 76% inhibition rate using a food poisoning technique. Topical application of SA-CNPs increased the activities of plant defence enzymes and antioxidant enzymes in tomato plants. In an in vitro study, the percent efficacy of disease control (PEDC) demonstrated that 0.1% and 0.15% SA-CNPs provided 50% and 45% efficacy, respectively, in controlling FOL infection in tomato plants. These findings confirm the efficacy of SA-CNPs in reducing Fusarium wilt by leveraging their antifungal properties and enhancing antioxidant and plant defence enzymes.
Graphical abstract











Similar content being viewed by others
Data availability
All data supporting the findings of this study are included within the manuscript.
References
Food and Agriculture Organization of the United Nations (2019) FAOSTAT Statistical Database
Snyder WC, Hansen HN (1940) The species concept in Fusarium. Am J Bot 1:64–67
Edel-Hermann V, Lecomte C (2019) Current status of Fusarium oxysporum formae speciales and races. Phytopathology 109:512–530
Walker JC (1971) Fusarium wilt of tomato. APS 56
Hubbeling N, Alexander LJ, Cirulli M (1971) Resistance to Fusarium and Verticillium wilts in tomato 589:1006–1016
Michielse CB, Rep M (2009) Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 10:311
McGrath DJ, Maltby JE (1989) Fusarium wilt race 3 resistance in tomato. Acta-Hortic 247:107–109
Malhotra SK, Vashistha RN (1993) Genetics of resistance to Fusarium wilt race 1 in current tomato (Lycopersicon pimpinellifolium). Indian J Agric Sci 63:246–347
Kirankumar R, Jagadeesh KS, Krishnaraj PU, Patil MS (2008) Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of Tobacco Mosaic Virus pathogen. J Agric Sci 21:309–311
Kapoor IJ (1988) Fungi involved in tomato wilt syndrome in Delhi, Maharashtra and Tamilnadu. Indian Phytopathol 41:208–213
Pandey KK, Gupta RC (2013) Virulence analysis of Fusarium oxysporum f. sp. lycopersici causing tomato wilt in India. J Mycol Plant Pathol 43:409–413
Asha BB, Chandra Nayaka S, Udayashankar AC, Srinivas C, Niranjana SR (2011) Biological control of Fusarium wilt of tomato. Int J Microbiol Res 3:79–84
Deshpande P, Dapkekar A, Oak MD, Paknikar KM, Rajwade JM (2017) Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr Polym 165:394–401
Meng X, Yang L, Kennedy JF, Tian S (2010) Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr Polym 81:70–75
Abd El-Gawad HG, Bondok AM (2015) Response of tomato plants to salicylic acid and chitosan under infection with tomato mosaic virus. Am Eur J Agric Environ Sci 15:1520–1529
Mandal S, Mallick N, Mitra A (2009) Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol Biochem 47:642–649
Achuo EA, Audenaert K, Meziane H, Höfte M (2004) The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol 53:65–72
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017) Cu-chitosan nanoparticle boost defence responses and plant growth in maize (Zea mays L.). Sci Rep 7:9754
Domard A, Domard M (2001) Chitosan: structure-properties relationship and biomedical applications. Int J Polym Mater 2:187–212
Kurita K (1998) Chemistry and application of chitin and chitosan. Polym Degrad Stab 59:117–120
Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, Raliya R, Biswas P (2016) Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J Agric Food Chem 64:6148–6155
Corradini E, De Moura MR, Mattoso LH (2010) A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym Lett 1:4
Kumar S, Ye F, Dobretsov S, Dutta J (2019) Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl Sci 9:2409
Manikandan A, Sathiyabama M (2016) Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int J Biol Macromol 84:58–61
Kheiri A, Jorf SM, Malihipour A, Saremi H, Nikkhah M (2017) Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int J Biol Macromol 102:526–538
Van SN, Minh HD, Anh DN (2013) Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal Agric Biotechnol 2:289–294
Nadendla SR, Rani TS, Vaikuntapu PR, Maddu RR, Podile AR (2018) HarpinPss encapsulation in chitosan nanoparticles for improved bioavailability and disease resistance in tomato. Carbohydr Polym 199:11–19
Muthukrishnan S, Murugan I, Selvaraj M (2019) Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr Polym 212:169–177
Kumaraswamy RV, Kumari S, Choudhary RC, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2019) Salicylic acid functionalized chitosan nanoparticle: a sustainable biostimulant for plant. Int J Biol Macromol 123:59–69
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013) Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683
Sathiyabama M, Charles RE (2015) Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium oxysporum f. sp. lycopersici. Carbohydr Polym 133:400–407
Vincent JM (1947) Distortion of fungal hyphae in the presence of certain inhibitors. Nature 159:850
Patil S, Sriram S, Savitha MJ (2011) Evaluation of non-pathogenic Fusarium for antagonistic activity against Fusarium wilt of tomato. Bio Control 25:118–123
Sumanta N, Haque CI, Nishika J, Suprakash R (2014) Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci 2231:606X
Luck H (1974) Estimation of Catalase Activity: Methods of Enzymology. Academic, New York
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Fridoch I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Mayer AM, Harel E, Shain Y (1964) 2,3-Naphthalenediol, a specific competitive inhibitor of phenolase. Phytochemistry 3:447–451
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25:111–123
Chester KS (1959) How sick is the plant. Plant Pathol 1:99–142
Wheeler BEJ (1969)An introduction to plant diseases. John Wiley, London
Chen Y, Mohanraj VJ, Wang F, Benson HA (2007) Designing chitosan-dextran sulfate nanoparticles using charge ratios. AAPS Pharm Sci Tech 8:131–139
Bodmeier R, Oh KH, Pramar Y (1989) Preparation and evaluation of drug-containing chitosan beads. Drug Dev Ind Pharm 15:1475–1494
Kumar S, Bhanjana G, Sharma A, Dilbaghi N, Sidhu MC, Kim KH (2017) Development of nanoformulation approaches for the control of weeds. Sci Total Environ 586:1272–1278
Sathiyabama M, Parthasarathy R (2016) Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr Polym 151:321–325
Yadav P, Yadav AB (2021) Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein/peptide-based drug delivery system. FJPS 7:1–9
Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F (2013) Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym 95:50–56
Rozana R, Yulizar Y, Saefumillah A, Apriandanu DO (2020) Synthesis, characterization and in vitro release study of efavirenz-loaded chitosan nanoparticle. AIP Conf Proc AIP Publishing 2242:1
Kee YJ, Zakaria L, Mohd MH (2020) Morphology, phylogeny and pathogenicity of Fusarium species from Sansevieria trifasciata in Malaysia. Plant Pathol 69:442–454
Anama YP, Díaz RA, Duarte-Alvarado D, Lagos-Burbano TC (2021) Morphological and pathogenic characterization of Fusarium oxysporum in lulo (Solanum spp.). Rev Fac Cienc Agrar 38:20–37
Srinivas C, Devi DN, Murthy KN, Mohan CD, Lakshmeesha TR, Singh B, Kalagatur NK, Niranjana SR, Hashem A, Alqarawi AA, Tabassum B (2019) Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity: A review. Saudi J Biol Sci 26:1315–1324
Liu Y, Zhang L, Li X, Zhang H, Zhang Y, Li J (2019) Chitosan nanoparticles loaded with salicylic acid induce systemic acquired resistance and suppress cucumber powdery mildew. Nanomater 9:114
Khan MA, Ali I, Akhtar M, Shahzad M, Akhtar MS (2017) Chitosan nanoparticles loaded with salicylic acid as a biocontrol agent against anthracnose disease of mango. JPP 99:257–264
Arora A, Gupta A, Kumar P (2018) Chitosan nanoparticles loaded with salicylic acid: An effective biocontrol agent against early blight of tomato. JPP 100:619–624
Maruyama Y, Yamoto N, Suzuki Y, Chiba Y, Yamazaki KI, Sato T, Yamaguchi J (2013) The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci 213:79–87
Takahashi H, Chen Z, Du H, Liu Y, Klessig DF (1997) Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J 11:993–1005
Kwok LY, Schlüter D, Clayton C, Soldati D (2004) The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol Microbiol 51:47–61
Zhang J, Li H, Xu B, Li J, Huang B (2016) Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front Plant Sci 7:1500
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Kang K, Niu Z, Zhang W, Wei S, Lv Y, Hu Y (2023) Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana. Plants 12:828
Lionetti V, Cervone F, Bellincampi D (2012) Methyl esterification of pectin plays a role during plant–pathogen interactions and affects plant resistance to diseases. J Plant Physiol 169:1623–1630
Banerjee K, Pramanik P, Maity A, Joshi DC, Wani SH, Krishnan P (2019) Methods of using nanomaterials to plant systems and their delivery to plants (Mode of entry, uptake, translocation, accumulation, biotransformation and barriers). Adv Phytonano- tech 2019:123–152
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139:847–856
Djalali Farahani-Kofoet R, Witzel K, Graefe J, Grosch R, Zrenner R (2020) Species-specific impact of Fusarium infection on the root and shoot characteristics of asparagus. Pathogens 9:509
Yang H, Luo P (2021) Changes in photosynthesis could provide important insight into the interaction between wheat and fungal pathogens. Int J Mol Sci 22:8865
Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, Thakur J (2022) How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants 28:485–504
Khairy AM, Tohamy MR, Zayed MA, Mahmoud SF, El-Tahan AM, El-Saadony MT, Mesiha PK (2022) Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi J Biol Sci 29:2199–2209
Kravanja G, Primozic M, Knez Z, Leitgeb M (2019) Chitosan-based (nano) materials for novel biomedical applications. Molecules 24:1960
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mesa, A., Mythatha, G.S.S. & Balli, R. Evaluation of the potential of topically applied salicylic acid-encapsulated chitosan nanoparticles to protect tomato against Fusarium wilt. Iran Polym J 33, 671–686 (2024). https://doi.org/10.1007/s13726-024-01283-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-024-01283-z