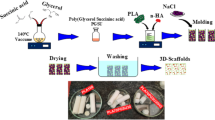
Abstract
In this work, asymmetrically poly(L-lactic acid)/poly(ethylene oxide) (PLLA/PEO) electrospun scaffolds were incorporated with novel mesoporous TiO2–alendronate nanoparticles loaded with dexamethasone (Dex–TiO2–ALN). The impact of nanoparticle incorporation on the fibers morphology and mechanical properties of the scaffolds was evaluated using FESEM technique and tensile stress assessments, respectively. After the investigation of the release behavior of the scaffolds, in vitro cell studies were performed employing MTT assay for cell viability assessment. Additionally, alkaline phosphatase (ALP) activity and calcium deposition assays were carried out for the potential determination of the osteogenic differentiation of the fabricated scaffolds on human adipose tissue-derived mesenchymal stem cells (hA-MSCs). The results showed that the incorporation of the nanoparticles decreased the average diameter of the electrospun fibers from 693 nm for the neat PLLA/PEO scaffold to 640 nm and 643 nm for the scaffolds containing TiO2–alendronate (TiO2–ALN) and Dex–TiO2–ALN nanoparticles, respectively. However, the surface morphology of the fibers did not changed significantly. According to the mechanical test results, the maximum strengths at breakpoint were 1.20, 1.27, and 1.28 MPa for the neat PLLA/PEO mat and its TiO2–ALN and Dex–TiO2–ALN-reinforced samples, respectively. The release profile showed an initial burst release around 11% of initial loaded Dex after 72 h, followed by a gradual increase to 38.23% at the end of the release period. Moreover, introducing Dex–TiO2–ALN nanoparticles improved cell viability, ALP activity, and calcium deposition as well as notified the importance of scaffold engineering for biomedical applications.
Graphical abstract







Similar content being viewed by others
Data availability
Not available.
References
Kong B, Sun W, Chen G, Tang C, Li M, Shao Z, Mi S (2017) Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci Rep 7:970. https://doi.org/10.1038/s41598-017-01072-0
Hosseinzadeh S, Zarei-Behjani Z, Bohlouli M, Khojasteh A, Ghasemi N, Salehi-Nik N (2022) Fabrication and optimization of bioactive cylindrical scaffold prepared by electrospinning for vascular tissue engineering. Iran Polym J 31:127–141
Liverani L, Raffel N, Fattahi A, Preis A, Hoffmann I, Boccaccini AR, Beckmann MW, Dittrich R (2019) Electrospun patterned porous scaffolds for the support of ovarian follicles growth: a feasibility study. Sci Rep 9:1150. https://doi.org/10.1038/s41598-018-37640-1
Birhanu G, Tanha S, Akbari Javar H, Seyedjafar E, Zandi-Karimi A, Kiani-Dehkordi B (2019) Dexamethasone loaded multi-layer poly-l-lactic acid/pluronic P123 composite electrospun nanofiber scaffolds for bone tissue engineering and drug delivery. Pharm Dev Technol 24:338–347. https://doi.org/10.1080/10837450.2018.1481429
Atila D, Hasirci V, Tezcaner A (2022) Coaxial electrospinning of composite mats comprised of core/shell poly (methyl methacrylate)/silk fibroin fibers for tissue engineering applications. J Mech Behav Biomed Mater 128:105105. https://doi.org/10.1016/j.jmbbm.2022.105105
Maleki H, Semnani Rahbar R, Nazir A (2020) Improvement of physical and mechanical properties of electrospun poly (lactic acid) nanofibrous structures. Iran Polym J 29:841–851. https://doi.org/10.1007/s13726-020-00844-2
Porgham-Daryasari M, Dusti Telgerd M, Karami MH, Zandi-Karimi A, Akbarijavar H, Khoobi M, Seyedjafari E, Birhanu G, Khosravian P, Sadat-Mahdavi F (2019) Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Artif Cells Nanomed Biotechnol 47:4020–4029. https://doi.org/10.1080/21691401.2019.1658594
Telgerd MD, Sadeghinia M, Birhanu G, Porgham-Daryasari M, Zandi-Karimi A, Sadeghinia A, Akbarijavar H, Karami MH, Seyedjafari E (2019) Enhanced osteogenic differentiation of mesenchymal stem cells on metal–organic framework based on copper, zinc, and imidazole coated poly-l-lactic acid nanofiber scaffolds. J Biomed Mater Res Part A 107:1841–1848. https://doi.org/10.1002/jbm.a.36707
Zhu X, Zhong T, Huang R, Wan A (2015) Preparation of hydrophilic poly (lactic acid) tissue engineering scaffold via (PLA)-(PLA-b-PEG)-(PEG) solution casting and thermal-induced surface structural transformation. J Biomater Sci Polym Ed 26:1286–1296. https://doi.org/10.1080/09205063.2015.1088125
Ju J, Peng X, Huang K, Li L, Liu X, Chitrakar Ch, Chang L, Gu Z, Kuang T (2019) High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer (Guildf) 180:121707. https://doi.org/10.1016/j.polymer.2019.121707
Wang H, Di J, Sun Y, Fu J, Wei Z, Matsui H, del C Alonso A, Zhou S, (2015) Biocompatible PEG-chitosan@ carbon dots hybrid nanogels for two-photon fluorescence imaging, near-infrared light/pH dual-responsive drug carrier, and synergistic therapy. Adv Funct Mater 25:5537–5547. https://doi.org/10.1002/adfm.201501524
Zhang C, Wang L, Zhai T, Wang X, Dan Y, Turng L-S (2016) The surface grafting of graphene oxide with poly (ethylene glycol) as a reinforcement for poly (lactic acid) nanocomposite scaffolds for potential tissue engineering applications. J Mech Behav Biomed Mater 53:403–413. https://doi.org/10.1016/j.jmbbm.2015.08.043
Xie H, He M, Deng X-Y, Du L, Fan C-J, Yang K-K, Wang Y-Z (2016) Design of poly (L-lactide)–poly (ethylene glycol) copolymer with light-induced shape-memory effect triggered by pendant anthracene groups. ACS Appl Mater Interfaces 8:9431–9439. https://doi.org/10.1021/acsami.6b00704
Moradikhah F, Doosti-Telgerd M, Shabani I, Soheili S, Dolatyar B, Seyedjafari E (2020) Microfluidic fabrication of alendronate-loaded chitosan nanoparticles for enhanced osteogenic differentiation of stem cells. Life Sci 254:117768. https://doi.org/10.1016/j.lfs.2020.117768
Soheili S, Mandegar E, Moradikhah F, Doosti-Telgerd M, Akbari-Javar H (2021) Experimental and numerical studies on microfluidic preparation and engineering of chitosan nanoparticles. J Drug Deliv Sci Technol 61:102268. https://doi.org/10.1016/j.jddst.2020.102268
Farahani M, Moradikhah F, Shabani I, Soflou RK, Seyedjafari E (2021) Microfluidic fabrication of berberine-loaded nanoparticles for cancer treatment applications. J Drug Deliv Sci Technol 61:102134. https://doi.org/10.1016/j.jddst.2020.102134
Heydariyan Z, Monsef R, Salavati-Niasari M (2022) Insights into impacts of Co3O4-CeO2 nanocomposites on the electrochemical hydrogen storage performance of g-C3N4: Pechini preparation, structural design and comparative study. J Alloys Compd 924:166564. https://doi.org/10.1016/j.jallcom.2022.166564
Monsef R, Salavati-Niasari M (2023) Architecturally robust tubular nano-clay grafted Li0.9Ni0.5Co0.5O2-x/LiFeO2 nanocomposites: new implications for electrochemical hydrogen storage. Fuel 332:126015. https://doi.org/10.1016/j.fuel.2022.126015
Salavati-Niasari M, Banitaba SH (2003) Alumina-supported Mn(II), Co(II), Ni(II) and Cu(II) bis(2-hydroxyanil)acetylacetone complexes as catalysts for the oxidation of cyclohexene with tert-butylhydroperoxide. J Mol Catal A Chem 201:43–54. https://doi.org/10.1016/S1381-1169(03)00128-6
Salavati-Niasari M, Farzaneh F, Ghandi M (2002) Oxidation of cyclohexene with tert-butylhydroperoxide and hydrogen peroxide catalyzed by alumina-supported manganese(II) complexes. J Mol Catal A Chem 186:101–107. https://doi.org/10.1016/S1381-1169(02)00045-6
Qiu K, Chen B, Nie W, Zhou X, Feng W, Wang W, Chen L, Mo X, Wei Y, He Ch (2016) Electrophoretic deposition of dexamethasone-loaded mesoporous silica nanoparticles onto poly(L-lactic acid)/poly(ε-caprolactone) composite scaffold for bone tissue engineering. ACS Appl Mater Interfaces 8:4137–4148. https://doi.org/10.1021/acsami.5b11879
Ma T-Y, Yuan Z-Y (2011) Metal phosphonate hybrid mesostructures: environmentally friendly multifunctional materials for clean energy and other applications. Chemsuschem 4:1407–1419. https://doi.org/10.1002/cssc.201100050
Liu S, Wu B, Yu Y, Shen Z (2019) Memory effect of arsenic-induced cellular response and its influences on toxicity of titanium dioxide nanoparticle. Sci Rep 9:107. https://doi.org/10.1038/s41598-018-36455-4
Crossland EJW, Noel N, Sivaram V, Leijtens T, Alexander-Webber JA, Snaith HJ (2013) Mesoporous TiO2 single crystals delivering enhanced mobility and optoelectronic device performance. Nature 495:215–219. https://doi.org/10.1038/nature11936
Reszka AA, Rodan GA (2003) Mechanism of action of bisphosphonates. Curr Osteoporos Rep 1:45–52. https://doi.org/10.1007/s11914-003-0008-5
Bai S-B, Liu D-Z, Cheng Y, Cui H, Liu M, Cui M-X, Mei Q-B, Zhou S-Y (2019) Osteoclasts and tumor cells dual targeting nanoparticle to treat bone metastases of lung cancer. Nanomedicine 21:102054. https://doi.org/10.1016/j.nano.2019.102054
Hochdörffer K, Abu Ajaj K, Schäfer-Obodozie C, Kratz F (2012) Development of novel bisphosphonate prodrugs of doxorubicin for targeting bone metastases that are cleaved pH dependently or by cathepsin B: synthesis, cleavage properties, and binding properties to hydroxyapatite as well as bone matrix. J Med Chem 55:7502–7515. https://doi.org/10.1021/jm300493m
Benyettou F, Rezgui R, Ravaux F, Jaber T, Blumer K, Jouiad M, Motte L, Olsen JC, Platas-Iglesias C, Magzoub M, Trabolsi A (2015) Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J Mater Chem B 3:7237–7245. https://doi.org/10.1039/x0xx00000x
Harmankaya N, Karlsson J, Palmquist A, Halvarsson M, Igawa K, Andersson M, Tengvall P (2013) Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta Biomater 9:7064–7073. https://doi.org/10.1016/j.actbio.2013.02.040
Jeon C, Oh KC, Park K-H, Moon HS (2019) Effects of ultraviolet treatment and alendronate immersion on osteoblast-like cells and human gingival fibroblasts cultured on titanium surfaces. Sci Rep 9:2581. https://doi.org/10.1038/s41598-019-39355-3
Motiei Pour M, Moghbeli MR, Larijani B, Akbari Javar H (2022) pH-Sensitive mesoporous bisphosphonate-based TiO2 nanoparticles utilized for controlled drug delivery of dexamethasone. Chem Pap 76:439–451. https://doi.org/10.1007/s11696-021-01870-x
Li H, Ma T-Y, Kong D-M, Yuan Z-Y (2013) Mesoporous phosphonate–TiO2 nanoparticles for simultaneous bioresponsive sensing and controlled drug release. Analyst 138:1084–1090. https://doi.org/10.1039/c2an36631b
Carbone R, Marangi I, Zanardi A, Giorgetti L, Chierici E, Berlanda G, Podestà A, Fiorentini F, Bongiorno G, Piseri P, Pelicci PG, Milani P (2006) Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials 27:3221–3229. https://doi.org/10.1016/j.biomaterials.2006.01.056
Kar A, Raja KS, Misra M (2006) Electrodeposition of hydroxyapatite onto nanotubular TiO2 for implant applications. Surf Coatings Technol 201:3723–3731. https://doi.org/10.1016/j.surfcoat.2006.09.008
Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmén A, Ellingsen JE (2006) Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted cp titanium endosseous implants. Biomaterials 27:926–936. https://doi.org/10.1016/j.biomaterials.2005.07.009
Seyedjafari E, Soleimani M, Ghaemi N, Shabani I (2010) Nanohydroxyapatite-coated electrospun poly(l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules 11:3118–3125. https://doi.org/10.1021/bm1009238
Karimi Z, Seyedjafari E, Mahdavi FS, Hashemi SM, Khojasteh A, Kazemi B, Mohammadi-Yeganeh S (2019) Baghdadite nanoparticle-coated poly l-lactic acid (PLLA) ceramics scaffold improved osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. J Biomed Mater Res A 107:1284–1293. https://doi.org/10.1002/jbm.a.36638
Ahmadi M, Seyedjafari E, Zargar SJ, Birhanu G, Zandi-Karimi A, Beiki B, Tuzlakoglu K (2017) Osteogenic differentiation of mesenchymal stem cells cultured on PLLA scaffold coated with Wharton’s Jelly. EXCLI J 16:785–794. https://doi.org/10.17179/excli2016-741
Kwon KC, Jo E, Kwon Y-W, Lee B, Ryu JH, Lee EJ, Kim K, Lee J (2017) Superparamagnetic gold nanoparticles synthesized on protein particle scaffolds for cancer theragnosis. Adv Mater 29:1701146. https://doi.org/10.1002/adma.201701146
Rungswang W, Kotaki M, Shimojima T, Kimura G, Sakurai S, Chirachanchai S (2014) Role of surfactant on inducing specific microdomains of block copolymer: an example case from polystyrene-b-poly(ethylene-co-1-butene)-b-polystyrene (SEBS) electrospun thermoplastic-elastomer fiber containing polyethylene glycol lauryl ether (PGLE). Polymer (Guildf) 55:2068–2076. https://doi.org/10.1016/j.polymer.2014.02.057
Alonso-Goulart V, Ferreira LB, Duarte CA, Lima IL, Ferreira ER, Oliveira BC, Vargas LN, Moraes DD, Silva IBB, de Oliveira FR, Souza AG, de Souza C-F (2018) Mesenchymal stem cells from human adipose tissue and bone repair: a literature review. Biotechnol Res Innov 2:74–80. https://doi.org/10.1016/j.biori.2017.10.005
Burrow KL, Hoyland JA, Richardson SM (2017) Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int 2017:2541275. https://doi.org/10.1155/2017/2541275
Yarak S, Okamoto OK (2010) Human adipose-derived stem cells: current challenges and clinical perspectives. An Bras Dermatol 85:647–656. https://doi.org/10.1590/S0365-05962010000500008
Yun Y-P, Kim S-J, Lim Y-M, Park K, Kim H-J, Jeong S-I, Kim SE, Song H-R (2014) The effect of alendronate-loaded polycarprolactone nanofibrous scaffolds on osteogenic differentiation of adipose-derived stem cells in bone tissue regeneration. J Biomed Nanotechnol 10:1080–1090. https://doi.org/10.1166/jbn.2014.1819
Acknowledgements
The authors appreciate the partial support from the Iran University of Science & Technology (IUST) and Tehran University of Medical Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motiei Pour, M., Moghbeli, M.R., Larijani, B. et al. Poly(L-lactic acid)/poly(ethylene oxide) electrospun scaffold containing dexamethasone-loaded TiO2–alendronate mesoporous nanoparticles utilized for bone tissue engineering application. Iran Polym J 32, 1179–1188 (2023). https://doi.org/10.1007/s13726-023-01194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01194-5