Abstract
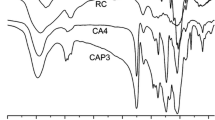
A highly rapid process is described for the preparation of cellulose triacetate and its effect on particle size and surface area of the product. The process involves microwave-assisted rapid synthesis of cellulose triacetate with very low amount of acetic anhydride (10–15% of acetic anhydride is used in conventional methods) in the presence of iodine as a catalyst using a designed reaction vessel. The technique used is simple and rapid; it is also characterized by a high conversion ratio (yield 100%). A small amount of iodine (115 and 230 mg, 1.15 and 2.3% of cellulose weight) was found to be effective in the production of cellulose triacetate using 25, 30 to 40 mL acetic anhydride for 10 g cellulose under microwave irradiation for 2–4 min. The production of cellulose triacetate and the degree of substitution were confirmed by FTIR, Raman, 1H NMR, and thermogravimetric analysis. The optimal reaction condition was discovered to be 3 min microwave radiation and 30 mL acetic anhydride in the presence of 230 mg iodine for 10 g cellulose. The effects of the amount of acetic anhydride, and amount of catalyst and reaction time on the specific surface area, pore volume, mean pore diameter, and particle size distribution were investigated. The highest surface area obtained was 39.63 m2/g. The specific surface area and particle size distribution are highly dependent on the amount of acetic anhydride and I2 catalyst. About 10% of the synthesized cellulose acetate showed particle size less than 200 nm.









Similar content being viewed by others
References
Das AM, Ali AA, Hazarika MP (2014) Synthesis and characterization of cellulose acetate from rice husk: eco-friendly condition. Carbohydr Polym 112:342–349
Gardner RM, Buchanan CM, Komarek RJ, Dorschel DD, Boggs C, White AW (1994) Compostability of cellulose acetate films. J Appl Polym Sci 52:1477–1488
Biswas A, Selling G, Appell M, Woods KK, Willett JL, Buchanan CM (2007) Iodine catalyzed esterification of cellulose using reduced levels of solvent. Carbohydr Polym 68:555–560
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688
Biswas A, Shogren RL, Willett JL (2005) Solvent-free process to esterify polysaccharides. Biomacromolecules 6:1843–1845
Yu D-G, Li X-Y, Wang X, Chian W, Liao Y-Z, Li Y (2013) Zero-order drug release cellulose acetate nanofibers prepared using coaxial electrospinning. Cellulose 20:379–389
Yu DG, Yu JH, Chen L, Williams GR, Wang X (2012) Modified coaxial electrospinning for the preparation of high-quality ketoprofen-loaded cellulose acetate nanofibers. Carbohydr Polym 90:1016–1023
Sassi JF, Chanzy H (1995) Ultra structural aspects of the acetylation of cellulose. Cellulose 2:111–127
Cerqueira DA, Filho GR, Meireles CS (2007) Optimization of sugarcane bagasse cellulose acetylation. Carbohydr Polym 69:579–582
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Yan L, Li W, Qi Z, Liu S (2006) Solvent-free synthesis of cellulose acetate by solid super acid catalysis. J Polym Res 13:375–378
Heinze T, Liebert T, Koschella A (2006) Esterification of polysaccharides. Springer, Berlin
Biswas A, Selling GS, Shogren RL, Willett JL, Buchanan CM, Cheng HN (2009) Iodine-catalyzed esterification of polysaccharides. Chem Today 27:4–6
Zhang X, Zhang W, Tian D, Zhou Z, Lu C (2013) A new application of ionic liquids for heterogeneously catalyzed acetylation of cellulose under solvent-free conditions. RSC Adv 3:7722–7725
Tian D, Han Y, Lu C, Zhang X, Yuan G (2014) Acidic ionic liquid as “quasi-homogeneous” catalyst for controllable synthesis of cellulose acetate. Carbohydr Polym 113:83–90
Cao J, Sun X, Lu C, Zhou Z, Zhang X, Yuan G (2016) Water-soluble cellulose acetate from waste cotton fabrics and the aqueous processing of all-cellulose composites. Carbohydr Polym 149:60–67
Yadav JS, Reddy BVS, Rao CV, Reddy MS (2003) Molecular iodine-catalyzed highly stereoselective synthesis of sugar acetylenes. Synth Stuttg 2:247–250
Phukan P (2004) Iodine as an extremely powerful catalyst for the acetylation of alcohols under solvent-free conditions. Tetrahed Lett 45:4785–4787
Biswas A, Saha BC, Lawton JW, Shogren RL, Willett JL (2006) Process for obtaining cellulose acetate from agricultural by-products. Carbohydr Polym 64:134–137
Yadav JS, Reddy BVS, Sengupta S, Guptal MK, Baishya G, Harshavardhanal SJ (2008) Iodine as a mild efficient and cost-effective catalyst for the synthesis of thiiranes from oxiranes. Monatsheftefür Chem Chem Mon 139:1363–1367
Behmadi H, Roshani M, Saadati SM (2009) Synthesis of phenanthrimidazole from 9,10-phenanthraquinone and aldehydes by molecular iodine as catalyst. Chin Chem Lett 20:5–8
Zhou Y, Yan PF, Li GM, Chen ZJ (2009) The synthesis application for iodine as a Lewis acid catalyst. Chin J Org Chem 29:1719–1727
Kartha KPR, Field RA (1997) Iodine: a versatile reagent in carbohydrate chemistry IV. Per-O-acetylation, regioselective acylation and acetolysis. Tetrahedron 53:11753–11766
Loupy A (2002) Microwaves in organic synthesis. Wiley-VCH, Weinheim
Li J, Zhang LP, Peng F, Bian J, Yuan TQ, Xu F, Sun R-C (2009) Microwave-assisted solvent-free acetylation of cellulose with acetic anhydride in the presence of iodine as a catalyst. Molecules 14:3551–3566
Xiong XQ, Cai L, Tang ZK (2012) Microwave-assisted click chemistry. Chin J Org Chem 32:1410–1428
Hu W, Chen S, Xu Q, Wang H (2011) Solvent-free acetylation of bacterial cellulose under moderate conditions. Carbohydr Polym 83:1575–1581
Hill CAS, Jones D, Strickland G, Centin NS (1998) Kinetic and mechanistic aspects of the acetylation of wood with acetic anhydride. Holzforschung 52:623–629
Sun RC, Tomkinson J, Ma PL, Liang SF (2000) Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydr Polym 42:111–122
Sun RC, Sun XF, Wen JL (2001) Fractional and structural characterization of lignins isolated by alkali and alkaline peroxide from barley straw. J Agric Food Chem 49:5322–5330
El Nemr A, Ragab S, El Sikaily A, Khaled A (2015) Synthesis of cellulose triacetate from cotton cellulose by using NIS as a catalyst under mild reaction conditions. Carbohydr Polym 130:41–48
El Nemr A, Ragab S, El Sikaily A (2016) Testing zinc chloride as a new catalyst for direct synthesis of cellulose di- and tri-acetate in a solvent free system under microwave irradiation. Carbohydr Polym 151:1058–1067
Kono H, Hashimoto H, Shimizu Y (2015) NMR characterization of cellulose acetate: chemical shift assignments, substituent effects, and chemical shift additivity. Carbohydr Polym 118:91–100
Nabili A, Fattoum A, Brochier-Salon M-C, Bras J, Elaloui E (2017) Synthesis of cellulose triacetate-I from microfibrillated date seeds cellulose (Phoenix dactylifera L.). Iran Polym J 26:137–147
Gregg SJ, Sing KSW (1982) Adsorption surface area and porosity. Academic Press, London
Rouquerol F, Rouquerol J, Sing KSW (1999) Adsorption by powders and porous solids. Academic Press, London
Acknowledgements
The authors are grateful to the Science and Technological Development Fund (STDF) of Egypt for its financial support of this work (Project Nos. 4788 and CB-4874).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemr, A.E., Ragab, S. & Sikaily, A.E. Rapid synthesis of cellulose triacetate from cotton cellulose and its effect on specific surface area and particle size distribution. Iran Polym J 26, 261–272 (2017). https://doi.org/10.1007/s13726-017-0516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-017-0516-2