Abstract
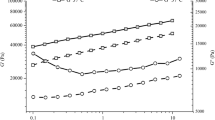
Natural polymers used as carrier materials in immobilization technology have the advantage of being non-toxic, biocompatible and biodegradable. In the present investigation, immobilization of yeast cells using different polymers has been carried out and the properties such as morphological, hardening, thermal stability and characterization of functional groups of alginate and hybrid beads (alginate–carrageenan and alginate–xanthan gum) have been studied by different techniques such as scanning electron microscope, texture analyzer, differential scanning calorimetry, and Fourier transfer infrared spectroscopy. The swelling behavior in terms of pH variation as well as flow properties of alginate and hybrid beads has also been examined. The hybrid beads prepared from alginate and carrageenan were found to be the best in terms of strength, cell holding capacity, pH and thermal stability. The reusability of beads was also studied in terms of enzyme activity of the entrapped yeast cells. The beads prepared by alginate–carrageenan were found to be more stable than alginate and alginate–xanthan beads. The yeast cells entrapped in alginate–carrageenan beads showed no significant decrease in enzyme activity up to seven batches. Thus, alginate–carrageenan beads can be used as a polymeric carrier/support to develop a stable and long-term immobilized cell system, which indicates its high potential for commercial applications in food and pharmaceutical sector.





Similar content being viewed by others
References
Klein J, Kressdorf B (1989) Polymers for the immobilization of whole cells and their application in biotechnology. Angew Makromol Chem 166:293–309
Chun KH, Kwon IC, Kim YH, La SB, Sohn YT, Jeong SY (1996) Preparation of sodium alginate microspheres containing hydrophilic β-lactam antibiotics. Arch Pharm Res 19:106–116
Kwon KH, Jung K-Y, Yeom SH (2009) Comparison between entrapment methods for phenol removal and operation of bioreactor packed with co-entrapped activated carbon and Pseudomonas fluorescence KNU417. Bioprocess Biosyst Eng 32:249–256
Hilge-Rotmann B, Rehm H-J (1991) Relationship between fermentation capability and fatty acid composition of free and immobilized Saccharomyces cerevisiae. Appl Microbiol Biotechnol 34:502–508
Jirku V (1999) Whole-cell immobilization as a means of enhancing ethanol tolerance. J Ind Microbiol Biotechnol 22:147–151
Hermann-Josef H, Keweloh H, Rehm H-J (1991) Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol 57:1213–1217
Farahani TD, Farahani EV, Mirzadeh H (2006) Swelling behaviour of alginate-N, O carboxymethyl chitosan gel beads coated by chitosan. Iran Polym J 15:405–415
Kafshgari MH, Khorram M, Mansouri M, Samimi A, Osfouri S (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J 21:99–107
Jain A, Gupta Y, Jain SK (2007) Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J Pharm Pharm Sci 10:86–128
Fundueanu G, Nastruzzi C, Carpov A, Desbrieres J, Rinaudo M (1999) Physico-chemical characterization of Ca-alginate microparticles produced with different methods. Biomaterials 20:1427–1435
Homayouni A, Ehsani MR, Azizi A, Yarmand MS, Razavi SH (2007) Effect of lecithin and calcium chloride solution on the microencapsulation process yield of calcium alginate beads. Iran Polym J 16:597–606
Mahdavinia GR, Massoudi A, Baghban A, Massoumi B (2012) Novel carrageenan-based hydrogel nanocomposites containing laponite RD and their application to remove cationic dye. Iran Polym J 21:609–619
Baets SD, Vandamme EJ, Steinbuchel A (eds) (2002) Biopolymers, Polysaccharides II, vol 6. Wiley, New York, pp 215–273
Whistler RL, Bemiller JN (eds) (1973) Industrial Gum. 2nd edn, Academic, New York, pp 49–114
Garcõa-Ochoa F, Santos VE, Casas JA, Gomez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18:549–579
Pelletier E, Viebke C, Meadows J, Williams PA (2001) A rheological study of the order–disorder conformational transition of xanthan gum. Biopolymers 59:339–546
Talukdar MM, Plaizier-Vercammen J (1993) Evaluation of xanthan gum as a hydrophilic matrix for controlled release dosage form preparations. Drug Dev Ind Pharm 19:1037–1046
Kumari S, Panesar PS, Bera MB, Singh B (2011) Permeabilization of yeast cells for β-galactosidase activity using mixture of organic solvents: a response surface methodology approach. Asian J Biotechnol 3:406–414
Marwaha SS, Kennedy JF (1984) Ethanol production from whey permeate by immobilized yeast cells. Enzyme Microb Technol 6:18–22
Mohamadnia Z, Jamshidi A, Mobedi H, Ahmadi E, Zohuriaan-Mehr MJ (2007) Full natural hydrogel beads for controlled release of acetate and disodium phosphate derivatives of betamethasone. Iran Polym J 16:711–718
Pongjanyakul T, Puttipipatkhachorn S (2007) Xanthan-alginate composite gel beads: molecular interaction and in vitro characterization. Int J Pharm 331:61–71
Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB (2005) Papain entrapment in alginate beads for stability improvement and site-specific delivery: physicochemical characterization and factorial optimization using neural network modeling. AAPS Pharm Sci Technol 6:209–222
Sevukarajan M, Nair KJKR, Jeyachitra B (2011) Preparation and characterization of hydrogel based drug delivery system of quetiapine fumarate. Int J Chem Pharm Sci 2:19–25
Dey G, Singh B, Banerjee R (2003) Immobilization of α-amylase produced by Bacillus circulans GRS 313. Braz Arch Biol Technol 46:167–176
Kim CJ, Lee PI (1992) Effect of loading on swelling-controlled drug release from hydrophobic polyelectrolyte gel beads. Pharm Res 9:1268–1274
Wan Ngah WS, Endud CS, Mayanar R (2002) Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym 50:181–190
Covarrubias SA, de-Bashan LE, Moreno M, Bashan Y (2012) Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl Microbiol Biotechnol 93:2669–2680
Rao BS, Pundle AV, Prabhune AA, Shankar V, Sivaraman H (1986) Ethanol production by yeast cells immobilized in open-pore agar. Appl Biochem Biotechnol 12:17–24
Zhang J, Xu S, Zhang S, Du Z (2008) Preparation and characterization of tamarind gum/sodium alginate composite gel beads. Iran Polym J 17:899–906
Pohlman NA, Severson BL, Ottino JM, Lueptow RM (2006) Surface roughness effects in granular matter: influence on angle of repose and the absence of segregation. Phys Rev E 73:031304
Badarinath AV, Reddy JRK, Rao KM, Alagusundaram M, Gnanaprakash K, Chetty CMS (2010) Formulation and characterization of alginate microbeads of flurbiprofen by ionotropic gelation technique. Int J Chem Tech Res 2:361–367
Minhas MU, Ahmad M, Ali L, Sohail M (2013) Synthesis of chemically cross-linked polyvinyl alcohol-co-poly(methacrylic acid) hydrogels by copolymerization; a potential graft-polymeric carrier for oral delivery of 5-fluorouracil. DARU J Pharm Sci 21:44
Siso MIG, Freire MA, Ramil E, Belmonte ER, Torres AR, Cerdan E (1994) Covalent immobilization of β-galactosidase on corn grits: a system for lactose hydrolysis without diffusional resistance. Process Biochem 29:7–12
Panesar PS (2007) Lactose hydrolysis in whole milk using immobilized Kluyveromyces marxianus cells. Am J Food Technol 2:288–294
Acknowledgments
The authors acknowledge Council of Scientific and Industrial Research (CSIR), New Delhi, India for the financial support given and Sant Longowal Institute of Engineering and Technology (SLIET), Longowal, India for providing laboratory facility to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumari, S., Panesar, P.S., Bera, M.B. et al. Comparative studies on physico-chemical characterization of yeast cells entrapped with alginate and hybrid beads. Iran Polym J 23, 111–119 (2014). https://doi.org/10.1007/s13726-013-0206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0206-7