Abstract
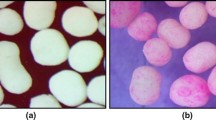
Polymeric mucoadhesive pellets of nifedipine were designed using computer software and they were prepared by extrusion-spheronization using HPMC K15M and κ-carrageenan with microcrystalline cellulose. A randomized rotatable two factor central composite design was applied for assessment of influence of two independent variables such as concentration of κ-carrageenan and HPMC K15M on dependent variables. Pellets were characterized by FTIR, DSC, SEM, flow properties, particle size, abrasion resistance, sphericity, drug content, percent production yield, in vitro drug release, ex vivo mucoadhesion, stability studies and similarity factor. The optimized formulation was selected based on criteria of sphericity nearest to 1.0 with maximum cumulative drug release percentage. Formulation NF6 exhibited sufficient porous spheres, free flowing and smooth surface mucoadhesion of 91.34 % and drug content 98.22 ± 0.37 %. Kinetic modeling revealed that the formulation followed the Higuchi model and showed the Quassi-Fickian drug release mechanism. The similarity factor, F2 value, was found to be 74 ± 6 and there was no significant change in drug content and ex vivo mucoadhesion after 90 days at 40 ± 2 °C, and 75 ± 5 % RH clearly indicated the optimized batch NF6 was stable. Thus, it can be concluded that use of κ-carrageenan, microcrystalline cellulose and HPMC K15M at the 20:35:10 w/w ratio could provide an effective carrier for enhancement of sphericity and sustained release of matrix pellets.






Similar content being viewed by others
References
Pund S, Joshi A, Vasu K, Nivsarkar M, Shishoo C (2011) Gastroretentive delivery of rifampicin: in vitro mucoadhesion and in vivo gamma-scintigraphy. Int J Pharm 411:106–112
Ige PP, Gattani SG (2012) Design and in vitro and in vivo characterization of mucoadhesive matrix pellets of metformin hydrochloride for oral controlled release: a technical note. Arch Pharm Res 35:487–498
Rouge N, Doelker E, Buri P (1996) Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int J Pharm 136:117–139
Klausner EA, Lavy E, Friedman M, Hoffman A (2003) Expandable gastroretentive dosage forms. J Control Rel 90:143–162
Moes AJ (1993) Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst 10:143–195
Gupta PK, Robinson JR (1992) Oral controlled-release delivery. In: Treatise on controlled drug delivery. Marcel Dekker, New York, 255–310
Huang J, Wigent RJ, Bentzley CM, Schwartz JB (2006) Nifedipine solid dispersion in microparticles of ammonio methacrylate copolymer and ethylcellulose binary blend for controlled drug delivery: effect of drug loading on release kinetics. Int J Pharm 319:44–54
Mounier-Vehier C, Bernaud C, Carre A, Lequeuche B, Hotton JM, Charpentier JC (1998) Compliance and antihypertensive efficacy of amlodipine compared with nifedipine slow-release. Am J Hypertens 11:478–486
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC (2006) A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 23:2709–2728
Chary RB, Rao YM (2000) Formulation and evaluation of methocel K15 M bioadhesive matrix tablets. Drug Dev Ind Pharm 26:901–906
Robert EO, Joseph BS (1989) Extrusion and spheronization technology. In: Issac, Ghebre-Sellassie (Ed) Pharmaceutical pelletization technology. Marcel Dekker, New York 37:187–216
Howard MA, Neaua SH, Sack MJ (2006) PEO and MPEG in high drug load extruded and spheronized beads that are devoid of MCC. Int J Pharm 307:66–76
Deasy PB, Gouldson MP (1996) In vitro evaluation of pellets containing enteric coprecipitates of nifedipine formed by non-aqueous spheronization. Int J Pharm 132:131–141
Bornhoft M, Thommes M, Kleinebudde M (2005) Preliminary assessment of carrageenan as excipient for extrusion/spheronisation. Eur J Pharm Biopharm 59:127–131
Thommes M, Kleinebudde M (2006) Use of κ-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. I. Influence of type and fraction of filler. Eur J Pharm Biopharm 63:59–67
Ghanam D, Hassan I, Kleinebudde P (2010) Compression behavior of κ-carrageenan pellets. Int J Pharm 390:117–127
Hausner HH (1967) Friction condition in mass of metal powders. Int J Powder Metall 3:7–13
Carr RL (1965) Classifying flow properties of solids. Chem Eng 18:163–168
Bouwmana AM, Bosma JC, Vonk P, Wesselingh JA, Frijlink HW (2004) Which shape factor(s) best describe granules? Powder Technol 146:66–72
Podczeck F, Rahman SR, Newton JM (1999) Evaluation of a standardised procedure to assess the shape of pellets using image analysis. Int J Pharm 192:123–138
Mortazavi SA (1995) An in vitro assessment of mucus/mucoadhesive interactions. Int J Pharm 124:173–182
Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR (2012) Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J Microencap 29:103–114
Badawy SIF, Shah KR, Surapaneni MS, Szemraj MM, Hussain M (2010) Effect of spray-dried mannitol on the performance of microcrystalline cellulose-based wet granulated tablet formulation. Pharm Dev Technol 15:339–345
Gui ZY, Wang H, Gao Y, Lu C, Cheng S (2012) Morphology and melt rheology of biodegradable poly(lactic acid)/poly(butylene succinate adipate) blends: effect of blend compositions. Iran Polym J 21:81–89
Ige PP, Gattani SG (2012) Development of low density microspheres of metformin hydrochloride using ethyl cellulose and HPMC K4 M: in vitro and in vivo characterization. Polym Plastic Technol Eng 51:1537–1544
Remunan C, Bretal MJ, Nunez A, Vila Jato JL (1992) Accelerated stability study of sustained-release nifedipine tablets prepared with Gelucire. Int J Pharm 80:151–159
Acknowledgments
Authors are thankful to J.B. Chemicals and Pharmaceuticals, Mumbai, India for providing gift sample of nifedipine. Authors would like to thank Dr. SJ Surana Principal, RC Patel Institute of Pharmaceutical Education & Research, Shripur, Maharashtra, India for providing the necessary facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ige, P.P., Rajput, P., Pardeshi, C. et al. Development of pellets of nifedipine using HPMC K15 M and κ-carrageenan as mucoadhesive sustained delivery system and in vitro evaluation. Iran Polym J 22, 911–921 (2013). https://doi.org/10.1007/s13726-013-0192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0192-9