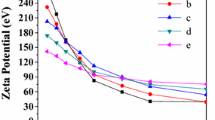
Abstract
Polyacrylamide gel (PAMG) method is a simple, fast and cheap method used for the synthesis of a wide variety of nanopowders. However, no adequate results have been reported on the thermal degradation behavior of PAMG which can be very effective on the final product properties. In this work, thermal degradation behavior of PAMG in the presence of TiCl4 as a precursor salt for synthesis of TiO2 nanoparticles was examined in comparison with linear polyacrylamide (LPAM) and pure PAMG by thermogravimetry/differential thermal analysis. Their thermal degradation kinetics was investigated, as well. The results showed that thermal degradation of all samples occurred in two stages at different onset temperatures. Despite the high thermal stability of pure PAMG compared to LPAM, the presence of TiCl4 as a mineral material in PAMG structure decreases the thermal degradation onset temperature, considerably. Furthermore for LPAM and PAMG, majority of weight loss occurs in the second stage, but in PAMG with TiCl4 the weight loss occurs mainly at the first stage. For more detailed investigation, residual materials were characterized by Fourier transform infrared spectroscopy and X-ray diffraction (XRD) techniques, attributing this trend to the presence of mineral materials in PAMG structure. XRD and transmission electron microscopy were also applied to confirm anatase crystalline structure and nanoscale distribution of the TiO2 particles synthesized via PAMG method.










Similar content being viewed by others
References
Kokabi M, Babaluo AA, Barati A (2006) Gelation process in low-toxic gelcasting systems. J Eur Ceram Soc 26:3083–3090
Tahmasebpour M, Babaluo AA, Razavi-Aghjeh MK (2008) Synthesis of zirconia nanopowders from various zirconium salts via polyacrylamide gel method. J Eur Ceram Soc 28:773–778
Liu H, Gong S, Hu Y, Zhao J, Liu J, Zheng Z, Zhou D (2009) Tin oxide nanoparticles synthesized by gel combustion and their potential for gas detection. Ceram Int 35:961–966
Tahmasebpour M, Babaluo AA, Shafiei S, Pipelzadeh E (2009) Studies on the synthesis of α-Al2O3 nanopowders by the polyacrylamide gel method. Powder Technol 191:91–97
Leung WM, Axelson DE, Van Dyke JD (1987) Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J Polym Sci Part A Polym Chem 25:1825–1846
Van Dyke JD, Kasperski KL (1993) Thermogravimetric study of polyacrylamide with evolved gas analysis. J Polym Sci Part A Polym Chem 31:1807–1823
Lee KE, Khan I, Morad N, Teng TT, Poh BT (2011) Thermal behavior and morphological properties of novel magnesium salt–polyacrylamide composite polymers. Polym Compos 32:1515–1522
Yang M-H (1998) The two-stage thermal degradation of polyacrylamide. Polym Test 17:191–198
Yang M-H (2002) On the thermal degradation of poly(styrene sulfone)s. V. Thermogravimetric kinetic simulation of polyacrylamide pyrolysis. J Appl Polym Sci 86:1540–1548
Yang M-H (2000) The thermal degradation of polyacrylamide with adsorbed metal ions as stabilizers. Polym Test 19:85–91
Órfão JJM, Martins FG (2002) Kinetic analysis of thermogravimetric data obtained under linear temperature programming—a method based on calculations of the temperature integral by interpolation. Thermochim Acta 390:195–211
Yao F, Wu Q, Lei Y, Guo W, Xu Y (2008) Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stab 93:90–98
Poletto M, Pistor V, Zeni M, Zattera AJ (2011) Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym Degrad Stab 96:679–685
Rao VK, Bardon MF, Stowe RA (1995) Kinetic parameters of composite propellants from thermogravimetric data. Combust Flame 102:219–225
Nalbandi A (2001) Kinetics of thermal degradation of polylactic acid under N2 atmosphere. Iran Polym J 10:371–376
Vyazovkin S, Wight CA (1997) Kinetics in solids. Annu Rev Phys Chem 48:125–149
Ma S, Hill JO, Heng S (1991) A kinetic analysis of the pyrolysis of some Australian coals by non-isothermal thermogravimetry. J Therm Anal Calorim 37:1161–1177
Fraga F, Núñez ER (2001) Activation energies for the epoxy system BADGE n = 0/m-XDA obtained using data from thermogravimetric analysis. J Appl Polym Sci 80:776–782
Azimi HR, Rezaei M, Abbasi F, Charchi A, Bahluli Y (2008) Non-isothermal degradation kinetics of MMA-St copolymer and EPS lost foams. Thermochim Acta 474:72–77
Azimi HR, Rezaei M, Abbasi F (2009) Thermo-oxidative degradation of MMA–St copolymer and EPS lost foams: kinetics study. Thermochim Acta 488:43–48
Peterson JD, Vyazovkin S, Wight CA (1999) Kinetic study of stabilizing effect of oxygen on thermal degradation of poly(methylmethacrylate). J Phys Chem 103:8087–8092
Wang H, Yang J, Long S, Wang X, Yang Z, Li G (2004) Studies on the thermal degradation of poly(phenylene sulfide sulfone). Polym Degrad Stab 83:229–235
Doğan F, Kaya İ, Bilici A (2011) Azomethine-based phenol polymer: synthesis, characterization and thermal study. Synth Met 161:79–86
Wang T-H, Navarrete-López AM, Li Sh, Dixon DA, Gole JL (2010) Hydrolysis of TiCl4: initial steps in the production of TiO2. J Phys Chem A 114:7561–7570
Acknowledgments
The authors wish to thank Sahand University of Technology (SUT) for financial support of this work. Also, thank coworkers and technical staff in nanostructure materials research center and institute of polymeric materials of SUT for their help during various stages of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakhshani, M., Kamrannejad, M.M., Babaluo, A.A. et al. Thermal degradation behavior and kinetic studies of polyacrylamide gel in TiO2 nanoparticles synthesis. Iran Polym J 21, 821–828 (2012). https://doi.org/10.1007/s13726-012-0086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-012-0086-2