Abstract
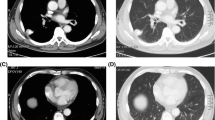
A 76-year-old man was diagnosed as having lung adenocarcinoma harboring an epidermal growth factor receptor (EGFR)-activating mutation based on a transbronchial biopsy specimen. Although laboratory data showed a marked elevation of saliva-type amylase activity in both the serum and urine, the salivary glands and pancreas were not clinically involved in hyperamylasemia. The patient was treated with EGFR tyrosine kinase inhibitors (EGFR-TKIs). However, the EGFR-TKIs were ineffective, and the patient’s amylase levels increased in parallel with disease progression. He eventually died of respiratory failure. On autopsy, the histological findings showed an invasive adenocarcinoma with micropapillary growth and an immunohistochemical study revealed the localization of the amylase in the cytoplasm of tumor cells. Using fluorescence in situ hybridization analyses of both transbronchial lung biopsy specimens obtained prior to treatment and the autopsied lung tumor, we confirmed the amplification of the MET gene prior to drug exposure. The result suggested that the lung cancer might have overcome the inhibition of EGFR-TKIs via MET amplification. This case report also indicated that the amylase levels of amylase-producing lung cancer vary with disease progression, and hyperamylasemia refractory to EGFR-TKIs may be a predictor of drug resistance to EGFR-TKIs. Therefore, if the amylase levels do not decrease sufficiently in response to EGFR-TKI treatment in patients with EGFR-mutated, amylase-producing lung cancer, the possibility that drug resistance to EGFR-TKIs may coexist with the EGFR mutation should be considered.



Similar content being viewed by others
References
Weiss MJ, Edmondson HA, Wertman M (1951) Elevated serum amylase associated with bronchogenic carcinoma. Am J Clin Pathol 21:1057–1061
Ammann RW, Berk JE, Fridhandler L et al (1973) Hyperamylasemia with carcinoma of the lung. Ann Intern Med 78:521–525
Lenler-Petersen P, Grove A, Brock A et al (1994) Alpha-amylase in resectable lung cancer. Eur Respir J 7:941–945
Ko HW, Tsai YH, Yu CT et al (2008) Good response to gefitinib for lung adenocarcinoma with hyperamylasemia: a case report. Chang Gung Med J 31:606–611
Zhang J, Zhang L, Pan S et al (2013) Amylase: sensitive tumor marker for amylase-producing lung adenocarcinoma. J Thorac Dis 5:167–169
Tsuda H, Akiyama F, Terasaki H et al (2001) Detection of HER-2/neu (c-erb B-2) DNA amplification in primary breast carcinoma. Interobserver reproducibility and correlation with immunohistochemical HER-2 overexpression. Cancer 92:2965–2974
Cappuzzo F, Marchetti A, Skokan M et al (2009) Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 27:1667–1674
Tsukawaki M, Izawa M, Yoshida M et al (1992) A case of amylase-producing lung cancer. Intern Med 31:60–63
Yamazaki S, Ebisawa S, Yasuo M et al (2007) Small-cell lung carcinoma produces salivary-type amylase: a case report with review. Intern Med 46:883–887
Tokumo M, Toyooka S, Kimura K et al (2005) The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 11:1167–1173
Inoue A, Suzuki T, Fukuhara T et al (2006) Prospective phase II study of gefitinib for chemotherapy-naïve patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24:3340–3346
Mitsudomi T, Yatabe Y (2007) Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 98:1817–1824
Kobayashi S, Boggon TJ, Dayaram T et al (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352:786–792
Kosaka T, Yatabe Y, Endoh H et al (2006) Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 12:5764–5769
Engelman JA, Zejnullahu K, Mitsudomi T et al (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039–1043
Cappuzzo F, Marchetti A, Skokan M et al (2009) Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 27:1667–1674
Yano S, Wang W, Li Q et al (2008) Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 68:9479–9487
Sordella R, Bell DW, Haber DA et al (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305:1163–1167
Tracy S, Mukohara T, Hansen M et al (2004) Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res 64:7241–7244
Nozu F, Owyang C, Tsunoda Y (2000) Involvement of phosphoinositide 3-kinase and its association with pp60src in cholecystokinin-stimulated pancreatic acinar cells. Eur J Cell Biol 79:803–809
Ikeda Y, Fukuoka SI (2003) Phosphatidic acid production, required for cholecystokinin octapeptide-stimulated amylase secretion from pancreatic acinar AR42 J cells, is regulated by a wortmannin-sensitive process. Biochem Biophys Res Commun 306:943–947
Yanagitani N, Kaira K, Sunaga N et al (2007) Serum amylase is a sensitive tumor marker for amylase-producing small cell lung cancer? Int J Clin Oncol 12:231–233
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nemoto, K., Hayashihara, K., Oh-ishi, S. et al. Lack of response to EGFR tyrosine kinase inhibitors in an amylase-producing lung cancer with a preexisting MET amplification. Int Canc Conf J 4, 236–240 (2015). https://doi.org/10.1007/s13691-015-0208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-015-0208-8