Abstract
Purpose of Review
The purpose of this review is to highlight recent research advances in noninvasive prenatal diagnostic methods.
Recent Findings
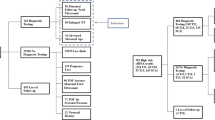
Recent studies developing noninvasive prenatal diagnostic (NIPD) methods have been focused on either fetal nucleated red blood cells (fNRBCs) or circulating trophoblasts (cTBs). Enriched cTBs were successfully utilized for whole genome profiling and short tandem repeat (STR) identification to confirm feto-maternal relationship. However, further analysis of isolated fNRBCs remains confined to examining fetal cytogenetics.
Summary
Invasive prenatal diagnostic procedures, amniocentesis, and chorionic villus sampling, are the gold standard for the diagnosis of fetal chromosomal abnormalities and genetic disorders. Meanwhile, noninvasive techniques of analyzing circulating cell-free fetal DNA (cffDNA) have been limited to screening tools and are highly fragmented and confounded by maternal DNA. By detecting circulating fetal nucleated cells (CFNCs) we are able to noninvasively confirm fetal chromosomal abnormalities, truly realizing the concept of “noninvasive prenatal diagnostics”. The primary technical challenge is the enrichment of the low abundance of CFNCs in maternal peripheral blood. For any cell-based NIPD method, both fetal whole genome profiling and confirmation of the feto-parental relationship are essential. This has been successfully performed using enriched and isolated cTBs, making cTB a better candidate for NIPD. cTB enumeration also correlates with abnormal fetal or placental development. On the other hand, downstream analysis of fNRBCs remains limited to examining fetal sex and aneuploidies. Furthermore, trophoblast-based NIPD via an endocervical sample is also promising because of reduced dilution from hematologic cells.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dimaio MS, Fox JE, Mahoney MJ. Prenatal diagnosis: cases and clinical challenges. 1st ed: Wiley; 2010.
Beckmann CRB, Herbert W, Laube D, Ling F, Smith R. Obstetrics and gynecology. Lippincott Williams & Wilkins ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
Society for Maternal-Fetal Medicine . Electronic address pso, Dugoff L, Norton ME, Kuller JA. The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol 2016;215(4):B2–B9. doi:https://doi.org/10.1016/j.ajog.2016.07.016.
Mujezinovic F, Alfirevic Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol. 2007;110(3):687–94. https://doi.org/10.1097/01.AOG.0000278820.54029.e3.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–7. https://doi.org/10.1016/S0140-6736(97)02174-0.
Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;105(51):20458–63. https://doi.org/10.1073/pnas.0810641105.
Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901. https://doi.org/10.1097/AOG.0b013e31824fb482.
Nicolaides KH, Syngelaki A, Gil M, Atanasova V, Markova D. Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X, and Y. Prenat Diagn. 2013;33(6):575–9. https://doi.org/10.1002/pd.4103.
Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–97. https://doi.org/10.1056/NEJMoa1407349.
Williams J 3rd, Rad S, Beauchamp S, Ratousi D, Subramaniam V, Farivar S, et al. Utilization of noninvasive prenatal testing: impact on referrals for diagnostic testing. Am J Obstet Gynecol. 2015;213(1):102 e1–6. https://doi.org/10.1016/j.ajog.2015.04.005.
Walknowska J, Conte FA, Grumbach MM. Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet. 1969;1(7606):1119–22.
• Beaudet AL. Using fetal cells for prenatal diagnosis: history and recent progress. Am J Med Genet C. 2016;172(2):123–7. https://doi.org/10.1002/ajmg.c.31487. Dr. Beaudet is an outstanding and leading researcher in the field of non-invasive prenatal diagnostics (NIPD). He gave a decent speech in the seminar, concluding the history and recent progress of NIPD. Everyone who would like to know the developmental hallmarks of NIPD should read this article.
Bianchi DW, Robert E. Gross lecture. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg. 2007;42(1):12–8. https://doi.org/10.1016/j.jpedsurg.2006.09.047.
Emad A, Bouchard EF, Lamoureux J, Ouellet A, Dutta A, Klingbeil U, et al. Validation of automatic scanning of microscope slides in recovering rare cellular events: application for detection of fetal cells in maternal blood. Prenat Diagn. 2014;34(6):538–46. https://doi.org/10.1002/pd.4345.
Kantak C, Chang CP, Wong CC, Mahyuddin A, Choolani M, Rahman A. Lab-on-a-chip technology: impacting non-invasive prenatal diagnostics (NIPD) through miniaturisation. Lab Chip. 2014;14(5):841–54. https://doi.org/10.1039/c3lc50980j.
Bhat NM, Bieber MM, Teng NN. One-step enrichment of nucleated red blood cells. A potential application in perinatal diagnosis. J Immunol Methods. 1993;158(2):277–80.
Kwon KH, Jeon YJ, Hwang HS, Lee KA, Kim YJ, Chung HW, et al. A high yield of fetal nucleated red blood cells isolated using optimal osmolality and a double-density gradient system. Prenat Diagn. 2007;27(13):1245–50. https://doi.org/10.1002/pd.1888.
Mavrou A, Kouvidi E, Antsaklis A, Souka A, Kitsiou Tzeli S, Kolialexi A. Identification of nucleated red blood cells in maternal circulation: a second step in screening for fetal aneuploidies and pregnancy complications. Prenat Diagn. 2007;27(2):150–3. https://doi.org/10.1002/pd.1640.
•• Kolvraa S, Singh R, Normand EA, Qdaisat S, van den Veyver IB, Jackson L, et al. Genome-wide copy number analysis on DNA from fetal cells isolated from the blood of pregnant women. Prenat Diagn. 2016;36(12):1127–34. https://doi.org/10.1002/pd.4948. These three articles are few representative, outstanding researches in the recent 5 years regarding NIPD using circulating trophoblasts (cTBs) as target cells. They were able to demonstrate sufficient sensitivity and specificity to recover TBs from maternal blood during the first trimester, and genetic information of the fetuses.
Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1979;76(3):1453–5.
de Wit H, Nabbe KC, Kooren JA, Adriaansen HJ, Roelandse-Koop EA, Schuitemaker JH, et al. Reference values of fetal erythrocytes in maternal blood during pregnancy established using flow cytometry. Am J Clin Pathol. 2011;136(4):631–6. https://doi.org/10.1309/AJCPHL3VXY0VMLXL.
He ZB, Guo F, Feng C, Cai B, Lata JP, He RX, et al. Fetal nucleated red blood cell analysis for non-invasive prenatal diagnostics using a nanostructure microchip. J Mater Chem B. 2017;5(2):226–35. https://doi.org/10.1039/c6tb02558g.
Mouawia H, Saker A, Jais JP, Benachi A, Bussieres L, Lacour B, et al. Circulating trophoblastic cells provide genetic diagnosis in 63 fetuses at risk for cystic fibrosis or spinal muscular atrophy. Reprod BioMed Online. 2012;25(5):508–20. https://doi.org/10.1016/j.rbmo.2012.08.002.
•• Breman AM, Chow JC, U’Ren L, Normand EA, Qdaisat S, Zhao L, et al. Evidence for feasibility of fetal trophoblastic cell-based noninvasive prenatal testing. Prenat Diagn. 2016;36(11):1009–19. https://doi.org/10.1002/pd.4924.These three articles are few representative, outstanding researches in the recent 5 years regarding NIPD using circulating trophoblasts (cTBs) as target cells. They were able to demonstrate sufficient sensitivity and specificity to recover TBs from maternal blood during the first trimester, and genetic information of the fetuses.
Ganshirt D, Smeets FW, Dohr A, Walde C, Steen I, Lapucci C, et al. Enrichment of fetal nucleated red blood cells from the maternal circulation for prenatal diagnosis: experiences with triple density gradient and MACS based on more than 600 cases. Fetal Diagn Ther. 1998;13(5):276–86. https://doi.org/10.1159/000020854.
Bianchi DW, Simpson JL, Jackson LG, Elias S, Holzgreve W, Evans MI, et al. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. Prenat Diagn. 2002;22(7):609–15. https://doi.org/10.1002/pd.347.
•• Hou S, Chen JF, Song M, Zhu Y, Jan YJ, Chen SH, et al. Imprinted NanoVelcro microchips for isolation and characterization of circulating fetal trophoblasts: toward noninvasive prenatal diagnostics. ACS Nano. 2017;11(8):8167–77. https://doi.org/10.1021/acsnano.7b03073. These three articles are few representative, outstanding researches in the recent 5 years regarding NIPD using circulating trophoblasts (cTBs) as target cells. They were able to demonstrate sufficient sensitivity and specificity to recover TBs from maternal blood during the first trimester, and genetic information of the fetuses.
Jain CV, Kadam L, van Dijk M, Kohan-Ghadr HR, Kilburn BA, Hartman C, et al. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Science translational medicine. 2016;8(363):363re4. https://doi.org/10.1126/scitranslmed.aah4661.
Vestergaard EM, Singh R, Schelde P, Hatt L, Ravn K, Christensen R, et al. On the road to replacing invasive testing with cell-based NIPT: five clinical cases with aneuploidies, microduplication, unbalanced structural rearrangement, or mosaicism. Prenat Diagn. 2017;37(11):1120–4. https://doi.org/10.1002/pd.5150.
Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014;47(10):2941–50. https://doi.org/10.1021/ar5001617.
Chen JF, Zhu Y, Lu YT, Hodara E, Hou S, Agopian VG, et al. Clinical applications of NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Theranostics. 2016;6(9):1425–39. https://doi.org/10.7150/thno.15359.
• Jan YJ, Chen JF, Zhu Y, Lu YT, Chen SH, Chung H, et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv Drug Deliv Rev. 2018;125:78–93. https://doi.org/10.1016/j.addr.2018.03.006. This review article concludes the mature performance of NanoVelcro platform in capturing and analyzing rare cells from peripheral blood, which contributes to the field of NIPD as well as circulating tumor cells (CTCs).
Wang S, Wang H, Jiao J, Chen KJ, Owens GE, Kamei K, et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew Chem Int Ed Engl. 2009;48(47):8970–3. https://doi.org/10.1002/anie.200901668.
Wang S, Liu K, Liu J, Yu ZT, Xu X, Zhao L, et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem. 2011;50(13):3084–8. https://doi.org/10.1002/anie.201005853.
Zhang N, Deng Y, Tai Q, Cheng B, Zhao L, Shen Q, et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv Mater. 2012;24(20):2756–60. https://doi.org/10.1002/adma.201200155.
Hou S, Zhao L, Shen Q, Yu J, Ng C, Kong X, et al. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew Chem. 2013;52(12):3379–83. https://doi.org/10.1002/anie.201208452.
Hou S, Zhao H, Zhao L, Shen Q, Wei KS, Suh DY, et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Adv Mater. 2013;25(11):1547–51. https://doi.org/10.1002/adma.201203185.
Fischer KE, Aleman BJ, Tao SL, Daniels RH, Li EM, Bunger MD, et al. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett. 2009;9(2):716–20.
Curtis ASG, Varde M. Control of cell behavior —topological factors. J Natl Cancer I. 1964;33(1):15.
Liu WF, Chen CS. Cellular and multicellular form and function. Adv Drug Deliv Rev. 2007;59(13):1319–28.
Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L, et al. Cytogenetic results from the U.S. collaborative study on CVS. Prenat Diagn. 1992;12(5):317–45.
Munne S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108(1):62–71 e8. https://doi.org/10.1016/j.fertnstert.2017.05.002.
Schattman GL. Chromosomal mosaicism in human preimplantation embryos: another fact that cannot be ignored. Fertil Steril. 2018;109(1):54–5. https://doi.org/10.1016/j.fertnstert.2017.11.022.
Huang A, Adusumalli J, Patel S, Liem J, Williams J 3rd, Pisarska MD. Prevalence of chromosomal mosaicism in pregnancies from couples with infertility. Fertil Steril. 2009;91(6):2355–60. https://doi.org/10.1016/j.fertnstert.2008.03.044.
Byeon Y, Ki CS, Han KH. Isolation of nucleated red blood cells in maternal blood for non-invasive prenatal diagnosis. Biomed Microdevices. 2015;17(6):118. https://doi.org/10.1007/s10544-015-0021-3.
Zhang H, Yang Y, Li X, Shi Y, Hu B, An Y, et al. Frequency-enhanced transferrin receptor antibody-labelled microfluidic chip (FETAL-Chip) enables efficient enrichment of circulating nucleated red blood cells for non-invasive prenatal diagnosis. Lab Chip. 2018;18(18):2749–56. https://doi.org/10.1039/c8lc00650d.
Chen F, Liu P, Gu Y, Zhu Z, Nanisetti A, Lan Z, et al. Isolation and whole genome sequencing of fetal cells from maternal blood towards the ultimate non-invasive prenatal testing. Prenat Diagn. 2017;37(13):1311–21. https://doi.org/10.1002/pd.5186.
Winter M, Hardy T, Rezaei M, Nguyen V, Zander-Fox D, Ebrahimi Warkiani M et al. Isolation of circulating fetal trophoblasts using inertial microfluidics for noninvasive prenatal testing. Adv Mater Technol. 2018;3(7). doi:https://doi.org/10.1002/admt.201800066.
Moser G, Drewlo S, Huppertz B, Armant DR. Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum Reprod Update. 2018;24(4):484–96. https://doi.org/10.1093/humupd/dmy008.
Imudia AN, Kumar S, Diamond MP, DeCherney AH, Armant DR. Transcervical retrieval of fetal cells in the practice of modern medicine: a review of the current literature and future direction. Fertil Steril. 2010;93(6):1725–30. https://doi.org/10.1016/j.fertnstert.2009.11.022.
Imudia AN, Suzuki Y, Kilburn BA, Yelian FD, Diamond MP, Romero R, et al. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum Reprod. 2009;24(9):2086–92. https://doi.org/10.1093/humrep/dep206.
Bolnick JM, Kilburn BA, Bajpayee S, Reddy N, Jeelani R, Crone B, et al. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil Steril. 2014;102(1):135–42 e6. https://doi.org/10.1016/j.fertnstert.2014.04.008.
Pfeifer I, Benachi A, Saker A, Bonnefont JP, Mouawia H, Broncy L, et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta. 2016;37:56–60. https://doi.org/10.1016/j.placenta.2015.11.002.
Falcidia E, Parano E, Grillo A, Pavone P, Takabayashi H, Trifiletti RR, et al. Fetal cells in maternal blood: a six-fold increase in women who have undergone amniocentesis and carry a fetus with down syndrome: a multicenter study. Neuropediatrics. 2004;35(6):321–4. https://doi.org/10.1055/s-2004-830365.
Parano E, Falcidia E, Grillo A, Takabayashi H, Trifiletti RR, Pavone P. Fetal nucleated red blood cell counts in peripheral blood of mothers bearing down syndrome fetus. Neuropediatrics. 2001;32(3):147–9. https://doi.org/10.1055/s-2001-16612.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Pin-Jung Chen, Pai-Chi Teng, Yazhen Zhu, Yu Jen Jan, Yalda Afshar, Li-Ching Chen, Margareta D. Pisarska, and Hsian-Rong Tseng declare no conflict of interest. Dr. Smalley reports personal fees from CytoLumina.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on High-risk Gestation and Prenatal Medicine
Rights and permissions
About this article
Cite this article
Chen, PJ., Teng, PC., Zhu, Y. et al. Noninvasive Prenatal Diagnostics: Recent Developments Using Circulating Fetal Nucleated Cells. Curr Obstet Gynecol Rep 8, 1–8 (2019). https://doi.org/10.1007/s13669-019-0254-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-019-0254-x