Abstract
Cytomegalovirus (CMV) occurs in 0.2 % to 2.2 % of all live births and is the most common cause of intrauterine infection and the leading infectious cause of sensorineural hearing loss and mental retardation. This article reviews literature that relate to the pathogenesis, diagnosis, and treatment of this disease for pregnant women and their fetus. Primary maternal CMV infection during pregnancy has a much higher rate of mother-to-fetus transmission and causes symptoms at birth and long-term disability than nonprimary infection. In addition, some research has shown that children with congenital CMV infection following first-trimester maternal infection are more likely to have severe sequelae. The prenatal diagnosis of fetal CMV infection includes serological testing (IgM detection and IgG avidity assay), amniocentesis, and ultrasound examination. The combination of the presence of CMV IgM antibodies and low CMV IgG avidity, along with maternal or fetal symptoms is used for the diagnosis of a primary maternal infection. Amniocentesis should be complemented until approximately 20-21 weeks of gestation to increase the sensitivity. Because ultrasound abnormalities are only found in less than 25 % of infected fetuses, ultrasound is as a relatively poor predictor of symptomatic congenital infection. CMV hyperimmunoglobulin also may be considered when the pregnant women are confirmed as primary CMV infection with low IgG avidity and amniotic fluid is found to contain CMV or CMV DNA. There is no consensus on the benefit of prenatal administration of ganciclovir into the umbilical vein.
Similar content being viewed by others
Introduction
Cytomegalovirus (CMV) is a member of the beta herpes virus and is considered lymphotropic. Its double-strand DNA includes approximately 160 open reading frames. A large portion of CMV genome encodes gene products that evade or interfere with host immune responses or interact with inflammatory molecules in ways that facilitate persistence and dissemination of CMV in the host [1]. Human fibroblasts, epithelial cells, and macrophages are permissive for CMV replication. The congenital, oral, and sexual routes, blood transfusion, and tissue transplantation are the major means by which CMV is transmitted. Activation and replication of CMV in the kidney and secretory glands promote its secretion in urine and body fluid, including throat washing, saliva, tears, breast milk, semen, stool, amniotic fluid, vaginal and cervical secretions, and tissues obtained for transplantation. In most cases, the virus replicates and is shed without causing symptoms. Initial infection with CMV can produce a heterophil-negative mononucleosis characterized by prolonged fever (>2 weeks), malaise, headache, pharyngitis, lymphadenopathy, hepatosplenomegaly, and arthralgias that cannot be distinguished clinically from mononucleosis due to Epstein-Barr virus [1]. However, primary CMV infection is almost always clinically silent in healthy children and adults, including pregnant women.
CMV establishes latent infection in mononuclear leukocytes and organs. Such infection reactivates by immunosuppression or after stimuli, such as inflammation or immune impairment because of disease or medical treatment. Hormonal changes associated with pregnancy also may stimulate reactivation of CMV. Immunosuppression during pregnancy may contribute to the increase in the incidence of primary or secondary CMV infections in pregnant women [2••].
Pathogenesis of Congenital CMV Infection
CMV is the leading cause of congenital infection, with morbidity and mortality at birth. Congenital CMV infection is considered to affect more children than other more commonly known conditions, such as Down’s syndrome, fetal alcohol syndrome, and spina bifida [1]. The burden of disease for congenitally infected infants includes intrauterine growth restriction, microcephaly, hepatosplenomegaly, petechiae, jaundice, chorioretinitis, thrombocytopenia, and anemia [2••]. In addition, congenital CMV infection can cause a variety of long-term disabilities, alone or in combination, such as mental retardation, learning disabilities, cerebral palsy, epilepsy, deafness or hearing impairment, and visual deficit or blindness [3].
Seroprevalence rates of CMV in women of reproductive age range from 40 % to 83 %, with women of lower socioeconomic status having a higher rate of previous infection [1]. CMV occurs in 0.2 % to 2.2 % of all live births and is the most common cause of intrauterine infection and the leading infectious cause of sensorineural hearing loss and mental retardation [2••].
There are two reasons to explain that CMV is the most common cause of maternal-fetal infection. First, CMV causes both primary and recurrent maternal infection. Second, CMV is highly prevalent in both developed countries, where primary maternal infection is common, and developing countries, where recurrent infections are most common [3].
At any time during pregnancy, primary or nonprimary maternal infection (including reactivation or reinfection with a different CMV strain) can lead to CMV crossing the placenta and infecting the fetus, resulting in congenital CMV infection. In the United States, approximately one-quarter of congenital CMV infections were attributable to primary maternal infection and three-quarters were attributable to nonprimary maternal infection [4]. Many women of reproductive age are seropositive for CMV and thus at risk for nonprimary rather than primary infection [1]. Besides, the rates of primary infection during pregnancy are low among seronegative women, because the pregnant duration is relatively short. Primary maternal infection during pregnancy has a much higher rate of mother-to-fetus transmission than does nonprimary infection (30-50 % vs. 1 %) [4]. It is comprehensible that maternal preconceptional immunity against CMV gives relatively good protection to fetus, so a smaller proportion becomes infected. Preexisting humoral immunity protects seropositive pregnant women against CMV reinfection at a rate of 66-93 % [3]. The fetal outcome after congenital CMV infection is summarized in Figure 1.
Additionally, primary infection appears to be more likely to cause symptoms at birth and long-term disability than nonprimary maternal infection. Berger et al. reported two cases of an acute primary CMV infection with no clinical signs of illness that was found in both mother and child and a recurrent CMV infection that resulted in necrotizing CMV encephalitis in the fetus [5].
CMV transmission rates appear to increase with advancing stages of pregnancy. Bodeus et al. reported that the transmission rate was 34.5 % during the first trimester, 44.1 % during the second trimester, and 73.3 % during the third trimester [6]. Enders et al. had reported that the rate of transmission increases gradually during gestation, based on 248 pregnant women with primary CMV infection. For the first, second, and third trimester of pregnancy, transmission rates were 30.1 %, 38.2 %, and 72.2 %, respectively [7].
Despite the higher transmission rate with maternal infection occurring later in pregnancy, the rate of sequelae in infected infant appears to be lower. CMV transmission in the first trimester is associated with the poorest fetal outcome [3]. Enders et al. indicated that the rate of symptomatically infected fetuses or newborns at birth was 22.8 % for any symptoms and 10.3 % for severe manifestation, but no symptoms were observed in infected newborns of mothers with primary infection in the preconceptional period and the third trimester [7].
In summary, research shows that children with congenital CMV infection following first trimester maternal infection are more likely to have severe sequelae, whereas CMV infection acquired during the third trimester is associated with a high rate of intrauterine transmission but a more favorable outcome for the infant.
Some studies found that the primary effect of antibodies is most likely on the placenta, which, during a primary CMV infection in the mother, becomes dysfunctional and results in poor oxygenation and nourishment of the fetus in utero [8]. Therefore, many symptoms of congenital CMV infection that are present at birth may not be due to any direct effect of the virus on the fetus but rather to the infection of placenta, which impairs its capacity to provide oxygen and nutrition to the developing fetus. Although not completely elucidated, placenta tissue damage is due to direct tissue injury by persistent CMV replication and immune complex deposition [3]. The increase in placental size occurs with primary maternal CMV infection, because the placental vasculature enlarges to compensate the fetus [8].
The useful effect of antibodies may be mediated through improved placental function and enhanced supplies of oxygen and nutrition to the fetus. Therefore, it has been recently observed that CMV hyperimmunoglobulin therapy is associated with reduced placental inflammation and size and fetal ultrasound abnormalities [8]. There is emerging evidence that viruses cause some stillbirths. Iwasenko et al. reported that the detection of CMV DNA in 15 % of fetal tissue or placenta from 130 stillbirths [9].
Additionally, CMV DNA was found in 27.4 % of fetal tissues from 73 cases of hydrops fetalis, spontaneous abortion, and unexplained fetal death in utero [10]. The significantly higher rate than other viruses suggests strong association between CMV infection in pregnancy and stillbirth. Virological markers are sought as potential prognostic factors to gauge the severity of congenital CMV infection. These markers include the viral load in neonatal blood and urine and genetic and immunologic variability. Some reports showed that CMV load in neonatal blood and urine seems to be related to symptomatic disease at birth and appears to be prognostic of adverse outcome [11].
The importance of the infecting CMV strain for clinical outcome has long been a matter of speculation. Genetic and immunologic variability may affect CMV virulence, irrespective of viral load. Some studies analyzing the correlation between CMV gN genotypes and clinical outcome have suggested that gN-1 could represent a less virulent virus phenotype, whereas the gN-4 group was predominantly associated with severe manifestations. These results suggest that gN genotypes might be markers for virulence of CMV wild-type strains and a discriminating factor for selection of CMV-infected newborns who are at risk of developing sequelae. Genotyping of viral strains in CMV-infected pregnant women may play a role when counseling families and early intervention for newborns [11].
Diagnosis of Congenital CMV Infection
Screening of Pregnant Women
Detection of serum IgG antibodies indicates that CMV infection occurred in the recent or distant past. The value of testing for IgG antibodies is to determine whether a patient has ever been infected by CMV. Serial retests in 2-4 weeks in a patient who is initially CMV IgG antibodies-negative must be achieved to identify seroconversion.
Diagnosis of Maternal Infection
Most CMV infections in pregnant women are asymptomatic even during the acute stage. Approximately 25 % of pregnant women with primary infection are reported to be symptomatic. Even in cases with symptoms, the manifestations are nonspecific and mild, such as persistent low fever, fatigue, and headache. Laboratory tests may sometimes disclose atypical lymphocytosis and slightly raised transaminase level [12•]. Clinical diagnosis of CMV infection is unreliable.
The pregnant women who are initially CMV IgG antibodies-negative can be diagnosed as primary infection when seroconversion occurred (from CMV IgG antibodies negative to positive). However, the amount of CMV IgG antibodies cannot to be used to predict accurately recent infection of CMV disease. The “gold standard” of primary CMV infection is maternal seroconversion or the presence of serum CMV IgM antibodies combined with low avidity CMV IgG antibodies. In fact, if consecutive blood samples are available, the presence of CMV IgM and IgG antibodies in a previously IgG-negative individual pregnant woman provides determination of seroconversion and primary CMV infection.
Testing for CMV IgM antibodies is the most widely used and appropriate procedure for screening pregnant women. Although CMV IgM antibodies occur in nearly all primary infection, they also may occur after reactivations or reinfections [13]. It is observed that CMV IgM usually peaks 3 to 6 months after a primary infection but may remain present in serum for more than 12 months [12•]. However, it is important to note that false-positive results are common and may arise in patients with other viral infections (e.g., parvovirus B19, Epstein-Barr virus) or autoimmune disease or as the result of interference by rheumatoid factor of the IgM class [14]. Hence, finding CMV IgM in a single serum of a pregnant woman does not alone establish a recent primary CMV infection during pregnancy [15].
Because of these difficulties with interpreting the serology, the IgG avidity assay can be a useful tool to assist in distinguishing primary infection from past or recurrent infection and can assist in dating the time of infection [12•]. Antibody avidity reflects the strength of binding between a polyvalent antigen and antibody. During the first week following primary infection, CMV IgG antibodies show a low avidity for the antigen, but they progressively and slowly mature, initially acquiring a moderate and then a high avidity. The high CMV avidity antibodies can be maintained for many years, but low avidity CMV IgG antibodies are found only after primary antigenic stimulation and usually last for approximately 16-18 weeks after the onset of CMV infection. The high avidity is detectable only with remote or recurrent CMV infection [12•]. An avidity index <30 % strongly suggests a primary infection of less than 3 months. The determination of CMV antibody avidity performed before 12-16 weeks of gestation is therefore a helpful tool to identify all pregnant women who may give birth to infected newborns (100 % sensitivity). If the IgG avidity index is determined later during pregnancy (after 18-20 weeks of gestation), the sensitivity is drastically reduced (62.5 %) [12•].
Currently, the combination of the presence of CMV IgM antibodies and low CMV IgG avidity, along with maternal or fetal symptoms, is used for the diagnosis of a primary maternal infection. In women whose pre-pregnancy serological status is unknown, the presence of high titers of serum-specific IgG with/without CMV IgM combined with high IgG avidity during the first 12-16 weeks of gestation may be indicative of a nonprimary CMV infection (recurrent infection or reinfection with different CMV strain). To date, serological diagnosis of nonprimary CMV infection is difficult and often unreliable, because no optimal diagnostic methods are available.
Many commercial assays are available, but there is considerable variation between these assays in sensitivity and specificity for detection of CMV IgM or IgG antibodies and CMV IgG avidity. There is not usually good agreement among these commercial assays for the detection of CMV IgM antibodies. This lack of concordance has been ascribed to the different assay principle (IgM-capture vs. indirect assay), to the employment of viral lysates vs. recombinant antigens, and to the assay threshold employed. Gentile et al. reported that the positivity rate for CMV IgM by AxSYM was significantly higher than other commercial assays [16]. Carlier et al. reported that Access had better sensitivity and specificity than VIDAS in detecting CMV IgG and IgM antibodies and concluded that Access CMV IgG and IgM tests were suitable for screening prenatal CMV infections [17]. Revello et al. reported the results of a comparative evaluation of eight CMV IgG avidity assays and found that these commercial kit performances for IgG avidity determination are variable [18].
Diagnosis of Fetal Infection
Once maternal infection has been documented, it is important to determine whether fetal infection also has occurred, because this will help to guide the need for further evaluation and surveillance during pregnancy. Therefore, fetal CMV infection should be closely monitored by ultrasound examination.
Ultrasound abnormalities in the fetus, including an increased maximal placental thickness, are independent predictors of a poor neonatal outcome [19]. Abnormal findings on ultrasound include intrauterine growth retardation, hydrops or ascites, hyperechogenic bowel, pleural or pericardial effusion, hepatosplenomegaly, intrahepatic calcinations, pseudoneconium ileus, and central nervous system abnormalities [12•]. In addition, these findings are only seen in less than 25 % of infected fetuses [2••]. Because lack of ultrasound findings does not exclude congenital infection, ultrasound is a relatively poor predictor of symptomatic congenital infection.
Compared with ultrasound findings, CMV isolation from amniotic fluid has a much higher sensitivity and specificity and is considered the “gold standard” for prenatal diagnosis of fetal CMV infection [12•]. The fetus excretes CMV in urine, so we may detect the virus in amniotic fluid when the fetus’s renal system is functional.
In addition, transmission of virus from mother to fetus may not occur shortly after maternal infection. Gestational age at time of amniocentesis has been shown to be an additional variable affecting sensitivity. Yinon et al. demonstrated a sensitivity of 30 % if the first amniotic fluid sample was taken before 21 weeks of gestation, increasing to 71 % thereafter. For these reasons, amniocentesis should be reserved until approximately 20-21 weeks of gestation and 6-8 weeks after maternal infection to avoid false-negative results [2••].
Viral culture of the amniotic fluid is 100 % specific but may yield false-negative results because of low sensitivity. Viral culture can take 2 weeks or longer to provide a result with a traditional viral culture system. Shell vial culture techniques have similar sensitivity compared with conventional CMV culture and allow detection of the virus in 24 to 48 hours after amniotic fluid collection [2••]. Some studies showed that PCR has near 100 % of sensitivity and specificity for detection of even small amounts of viral DNA in the amniotic fluid correlate with congenital infection at birth [2••, 12, 20]. For maximal accuracy, both viral culture and PCR should be obtained. A diagnosis of fetal CMV alone is insufficient to predict newborn disease.
A large amount of virus as measured by PCR in the amniotic fluid is most likely related to gestational age and should not be used as an independent predictor of a poor fetal outcome [20]. If both CMV culture and PCR are negative, then fetal infection can be ruled out with a high degree of certainty. Positive results in amniotic fluid identify CMV-infected fetuses but do not discriminate those infants who will have symptoms at birth [21].
In addition, low viral load in amniotic fluid sampled at 20-21 weeks of gestation and when the time interval between onset of maternal infection is ≥6–8 weeks is consistently found to be associated with asymptomatic congenital infection and can rule out fetal damage at birth. Although the highest median values of CMV DNA in amniotic fluid tend to indicate an increased risk of severe infection, the correlation between the high CMV load in amniotic fluid and fetal/neonatal outcome has not been demonstrated [21]. Amniocentesis may be used in cases of primary or secondary maternal CMV infection. The risk of fetal infection is low with secondary maternal infection; however, severe sequelae occasionally occurred. Therefore, the risk/benefit ratio of performing this invasive diagnostic test must be evaluated cautiously in case of secondary infection.
Cordocentesis may be a useful adjunct to amniocentesis, because fetal blood can be tested for detection of viral genome, IgM, and culture. PCR on fetal blood taken at 20-21 weeks of gestation has sensitivity for congenital infection lower than that obtained with amniotic fluid [12•]. The sensitivity of PCR on fetal blood reaches 100 % when the fetal blood is taken after 30 weeks of gestation [22]. The sensitivity of CMV IgM detection in fetal blood obtained after 20 weeks of gestation range from 55.5 % to 80 %. Moreover, the presence of CMV IgM represented a prognostic marker of congenital CMV disease. Yinon et al. studied 63 pregnant women with primary CMV infection and found no case of positive fetal serum IgM with negative amniotic fluid culture or PCR. These results indicated that cordocentesis did not increase the ability to accurately diagnose intrauterine infection [2••]. Umbilical cord sampling, however, has an increased procedural morbidity, whereas amniocentesis is safer.
Diagnosis of Newborn Infection
At birth, the newborn born of a mother with primary CMV infection during pregnancy should be tested for congenital infection. Detection of CMV in the urine or saliva within first 2 weeks of life by viral culture or PCR is useful to diagnose congenital CMV infection. Besides, newborn CMV IgM antibodies are only present in between 20 % and 70 % [12•]. All infant with congenital CMV infection should be evaluated carefully for hearing loss, retinitis, and neurological function and undergo follow-up monitoring at 1, 3, 6, and 12 months of life and annually thereafter until school age.
Treatment of Congenital CMV Infection
A report has described the administration of ganciclovir (or its oral form, valganciclovir) to pregnant women with fetal CMV infection. The result indicated that the pregnant women had a significant decrease of viral load in amniotic fluid, but the fetuses had no different outcome. The rate of neonatal disease for treated and untreated fetuses was 52 % vs. 58 % [23]. Another study reported that a pregnant woman with CMV infection had complete clearance of the virus from the amniotic fluid and subsequently had a healthy neonate with the use of prenatal ganciclovir [24]. Prenatal administration of ganciclovir into the umbilical vein also has been reported, but its value in improving the prognosis is not well established. Although the treatment with ganciclovir in pregnancies complicated by CMV infection could be very valuable, the potential toxicity and side effects of the drug must be further assessed.
Many studies supported the likely efficacy of CMV hyperimmunoglobulin as a treatment for pregnant women infected with CMV during pregnancy [8, 25]. The use of CMV hyperimmunoglobulin for treatment was evaluated in 157 pregnant women with primary CMV infection. It was found that 16 % of women treated with CMV hyperimmunoglobulin had infants with congenital CMV infection, and in comparison, 40 % of women who did not receive CMV hyperimmunoglobulin had infected infants. In addition, no adverse effects of the CMV hyperimmunoglobulin were observed [2••]. CMV hyperimmunoglobulin also may be considered when the pregnant women are confirmed as primary CMV infection with low IgG avidity and amniotic fluid is found to contain CMV or CMV DNA.
Conclusions
Congenital CMV infection is an important concern in pregnant women. Routine serologic testing during pregnancy is now performed in most countries for rubella, syphilis, HBV, and HIV. Thus, adding CMV to ongoing serologic testing is feasible.
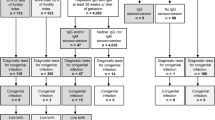
A flowchart for screening and diagnosis of CMV infection during pregnancy is recommended in Figure 2. In fact, diagnosis of CMV infection is more complex in women who are unaware of their serological status before pregnancy. Because most infections are asymptomatic, the only way to reveal primary infection is to implement specific serological testing before weeks 12-16 of gestation. In the case of seronegative women, serological testing for CMV needs to be repeated during pregnancy to rule out seroconversion. Arguments against screening include the fact that there is no effective vaccine and no treatment that has been proven effective.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Johnson J, Anderson B, Pass RF. Prevention of maternal and congenital cytomegalovirus infection. Clin Obstet Gynecol. 2012;55:521–30.
•• Yinon Y, Farine D, Yudin MH. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstet Gynecol Surv. 2010;65:736–43. This reference is very important because it made a complete description about the management of CMV infection in pregnancy.
Nigro G, Adler SP. Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol. 2011;23:123–8.
Wang C, Zhang X, Bialek S, et al. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Infect Dis. 2011;52:e11–3.
Berger A, Reitter A, Harter PN, et al. Problems and challenges in the diagnosis of vertical infection with human cytomegalovirus (CMV): lessons from two accidental cases. J Clin Virol. 2011;51:285–8.
Bodeus M, Zech F, Hubinont C. Human cytomegalovirus in utero transmission: follow-up of 524 maternal seroconversions. J Clin Virol. 2010;47:201–2.
Enders G, Daiminger A, Bäder U, et al. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol. 2011;52:244–6.
Maidji E, Nigro G, Tabata T, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol. 2010;177:1298–310.
Iwasenko JM, Howard J, Arbuckle S, et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis. 2011;203:1526–33.
Al-Buhtori M, Moore L, Benbow EW, et al. Viral detection in hydrops fetalis, spontaneous abortion, and unexplainedfetal death in utero. J Med Virol. 2011;83:679–84.
Pignatelli S, Lazzarotto T, Gatto MR, et al. Cytomegalovirus gN genotypes distribution among congenitally infected newborns and their relationship with symptoms at birth and sequelae. Clin Infect Dis. 2010;51:33–41.
• Lazzarotto T, Guerra B, Gabrielli L, et al. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect. 2011;17:1285–93. This article is important in that authors described the serological diagnosis of maternal CMV infection in detail.
De Paschale M, Agrappi C, Manco MT, et al. Positive predictive value of anti- HCMV IgM as an index of primary infection. J Virol Methods. 2010;168:121–5.
De Carolis S, Santucci S, Botta A. False-positive IgM for CMV in pregnant women with autoimmune disease: a novel prognostic factor for poor pregnancy outcome. Lupus. 2010;19:844–9.
De Paschale M, Agrappi C, Manco MT, et al. Positive predictive value of anti-HCMV IgM as an index of primary infection. J Virol Methods. 2010;168:121–5.
Gentile M, Galli C, Pagnotti P, et al. Measurement of the sensitivity of different commercial assays in the diagnosis of CMV infection in pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28:977–81.
Carlier P, Harika N, Bailly R, et al. Laboratory evaluation of the new Access® cytomegalovirus immunoglobulin IgM and IgG assays. J Clin Virol. 2010;49:192–7.
Revello MG, Genini E, Gorini G, et al. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J Clin Virol. 2010;48:255–9.
Vauloup-Fellous C, Picone O, Cordier AG, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46:S49–53.
Goegebuer T, Van Meensel B, Beuselinck K, et al. Clinical predictive value of real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples. J Clin Microbiol. 2009;47:660–5.
Lazzarotto T, Gabrielli L, Baccolini F. Prenatal diagnosis of congenital cytomegalovirus (CMV) infection and outcome in 598 pregnant women undergoing a primary CMV infection. In Abstracts of the 12th International CMV/BetaHerpesvirus Workshop, May 10–14 2009, Boston-USA.
Benoist G, Salomon LJ, Mohlo M, et al. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG. 2008;115:823–9.
Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valacyclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113–21.
Puliyanda DP, Silverman NS, Lehman D, et al. Successful use of oral ganciclovir for the treatment of intrauterine cytomegalovirus infection in a renal allograft recipient. Transpl Infect Dis. 2005;7:71–4.
Adler SP, Nigro G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol. 2009;46:S54–7.
Disclosures
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tung, YC., Lu, PL., Ke, LY. et al. CMV Infection and Pregnancy. Curr Obstet Gynecol Rep 1, 216–222 (2012). https://doi.org/10.1007/s13669-012-0028-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-012-0028-1