Abstract
Bioassay-guided fractionation of the EtOH extract from the flowers of Aquilaria sinensis (Lour.) Spreng. (Thymelaeaceae) led to the isolation of a new cucurbitane-type triterpenoid, aquilarolide A (1), along with five known compounds (2–6). The structure of 1 was elucidated by extensive 1D and 2D nuclear magnetic resonance (NMR) experiments and mass spectrometry (MS) data and theoretical calculations of its electronic circular dichroism (ECD) spectra. Aquilarolide A, cucurbitacin E (3), cucurbitacin B (4), and 7-hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one (6) showed significant cytotoxicity against human lung adenocarcinoma SPC-A-1, human lung squamous cell carcinoma NCI-H520, human lung adenocarcinoma A549, and paclitaxel-resistant A549 (A549/Taxol) cell lines. All four active compounds, with IC50 values ranging from 0.002 to 0.91 μM, had better inhibitory activities against A549/Taxol cells than paclitaxel (IC50 = 1.80 μM). Among them, cucurbitacin E (IC50 = 0.002 μM) is the most active. Further studies are needed to evaluate their in vivo antitumor activities and to clarify their mechanisms.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a major public health problem and one of the leading causes of mortality and morbidity worldwide [1]. Chemical drugs, such as paclitaxel, are useful to treat cancers. However, resistance to paclitaxel reduces the efficacy of chemotherapy and limits its clinical application [2]. Therefore, it is necessary to develop novel and effective therapeutic medicines or adjuvants for cancer. Natural plant resources are a rich source of anticancer agents. The discovery of new effective cancer drugs and understanding their underlying mechanism is one of the most studied topics among biologists and chemists.
Aquilaria sinensis (Lour.) Spreng. (Thymelaeaceae) is widely distributed in Hainan, Fujian, Yunnan, Guangdong, and Taiwan in China [3]. It has a particular economic interest because it is the principal source of agarwood (chen-xiang in Chinese), namely, the resinous heartwood of the plant. As a traditional Chinese medicine, agarwood has been widely investigated [4]. However, there have been only a few studies on the chemical constituents and bioactivities of A. sinensis flowers. The volatile constituents from flowers of A. sinensis have been analyzed by GC–MS [5]. Flavonoids and their glycosides are found in its flowers [6]. Benzophenone glycosides have been isolated from the flower buds of A. sinensis and two compounds, aquilasides B and C, displayed moderate cytotoxicity against SK-MEL cells with IC50 values of 17.0 and 12.0 μM, respectively [7].
In our screening for anticancer extracts of plants, the extract (PXS65) of A. sinensis flowers was found to possess significant inhibitory activities against 16 cancer cell lines (Table 1), especially inhibiting lung cancer cell lines, such as human lung adenocarcinoma SPC-A-1 (IC50 = 0.11 μg/mL), human lung squamous cell carcinoma NCI-H520 (IC50 = 0.25 μg/mL), and human lung adenocarcinoma A549 (IC50 = 0.44 μg/mL) cells. Then, a bioassay-guided isolation of cytotoxic constituents against A549, NCI-H520, SPC-A-1, paclitaxel-resistant A549 (A549/Taxol), and human normal bronchial epithelial BEAS-2B cell lines was conducted (Fig. 1; Tables 2–5), which led to the isolation of four active compounds, including a new cucurbitane-type triterpenoid (1) (Fig. 2). The bioassay results and the structural elucidation of aquilarolide A (1) are reported.
2 Results and discussion
2.1 Bioactivity-guided fractionation and isolation
The 90% EtOH extract (PXS65) of A. sinensis flowers after water extraction was tested in vitro for its cytotoxicity against 16 cancer cell lines, including SPC-A-1, NCI-H520, A549, human cervical cancer HeLa, human neuroblastoma SH-SY5Y, human ovarian carcinoma SK-OV-3, human T-cell leukemia MT4, human prostate cancer PC-3, human hepatoma SMMC-7721, human breast cancer MDA-MB-231, human small cell lung cancer NCI-H446, human large cell lung carcinoma NCI-H460, human colon cancer SW-480, human breast cancer MCF-7, human leukemia HL-60, and human colon cancer Caco2 cell lines, as well as the normal BEAS-2B cell line by 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) assay and their IC50 values (μg/mL) were determined (Table 1). PXS65 possessed pronounced cytotoxic activity against SPC-A-1, NCI-H520, A549, HeLa, SH-SY5Y, SK-OV-3, MT4, and PC-3 cells with IC50 values less than 1 μg/mL. Meanwhile, PXS65 exhibited weak inhibitory activity against SW480, MCF-7, HL-60, HL-60, Caco2, and BEAS-2B cells with IC50 values greater than 20 μg/mL. These data indicated that PXS65 had some selectivity for different cancerous cell lines and the normal BEAS-2B cell line. It had better inhibition against lung cancer cell lines than against other cancer cell lines (Table 1). Thus, the next bioactivity-guided separations were conducted according to the cytotoxicities of the fractions against lung cancer cell lines (A-549, NCI-H520, and SPC-A-1), as well as normal BEAS-2B cells and paclitaxel-resistant lung cancer A549/Taxol cells.
As shown in Table 2, the EtOAc-soluble fraction (PXS66-2) showed the most inhibitory activities against A-549 (IC50 = 0.17 μg/mL), NCI-H520 (IC50 = 0.08 μg/mL), SPC-A-1 (IC50 = 0.08 μg/mL), and A549/Taxol (IC50 = 0.08 μg/mL) cells. The inhibitory activity against A549/Taxol cells was better than that of paclitaxel (IC50 = 0.54 μg/mL), with lower toxicity (IC50 = 4.48 μg/mL) than that of paclitaxel (IC50 = 1.85 μg/mL) against normal BEAS-2B cells.
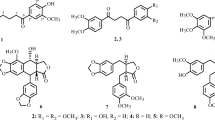
PXS66-2 was fractionated by silica gel column chromatography to yield six further fractions (B-1 to B-6), which were also submitted to a cytotoxicity assay (Table 3). Fr. B-1 showed observably higher inhibitory activities against these four lung cancer cell lines than other fractions with IC50 values less than or equal to 0.02 μg/mL. Fr. B-1 was separated by reverse-phase (RP) C18 silica gel column chromatography to yield 12 further fractions (B-1-1 to B-1-12), which were also submitted to a cytotoxicity assay (Table 4). Frs. B-1-6 and B-1-7 showed observably higher inhibitory activity against these four lung cancer cell lines than other fractions with IC50 values less than or equal to 0.02 μg/mL. Next, Frs. B-1-6 and B-1-7 were isolated and purified to yield six compounds (1–6) (Fig. 2).
2.2 Structural elucidation of isolates 1–6
In total, six secondary metabolites (Fig. 2), including a new metabolite (1), were isolated from the cytotoxically active fractions of A. sinensis flowers as a result of chromatographic separations. The chemical structure of the new compound was elucidated by 1D and 2D nuclear magnetic resonance (NMR) experiments as well as high-resolution electron ionization mass spectrometry (HRESIMS) and electronic circular dichroism (ECD) calculations.
Compound 1 was isolated as a white amorphous powder and exhibited a quasi-molecular ion peak at m/z 567.2937 [M + Na]+ in HRESIMS, suggesting a molecular formula of C31H44O8 (calcd. for C31H44NaO8, 567.2934) and 10 degrees of unsaturation. The 1H NMR spectrum (Table 1) of 1 indicated the presence of nine methyl groups at δH 0.98, 1.05, 1.33, 1.42, 1.51, 1.53, 1.54, 1.57, and 2.02 (methyl protons of an acetyl group) ppm, a trans double bond at δH 7.05 (d, J = 15.7 Hz) and 6.44 (d, J = 15.7 Hz) ppm, and a trisubstituted double bond at δH 5.75 (br s) ppm. The 13C NMR spectrum (Table 1) of 1 displayed 31 carbon signals indicating the presence of four carbonyl groups (δC 211.7, 202.4, 172.0, and 170.3), two double bonds (δC 152.1, 136.7, 120.6, and 120.2), nine methyl groups (δC 31.5, 30.6, 26.5, 25.9, 23.9, 22.0, 19.9, 18.5, and 18.4), four methylenes, four sp3 methines, and six quaternary carbons. These data showed a similar signal pattern with those of a lactone-type norcucurbitacin, neocucurbitacin E, except for the double bond at Δ23 in 1 [8].
Based on the 1H–1H COSY correlations (Fig. 3), four connections, H2-1/H-10, H-6/H2-7/H-8, H2-15/H-16/H-17, and H-23/H-24, were deduced. The HMBC data revealed the lactone-type structure of ring A, similar to that of neocucurbitacin E [8], since H2-1 (δ 2.50 and 2.16) was correlated to the carbon atoms at δC = 172.0 (C-2), 136.7 (C-5), and 47.7 (C-9) ppm, as well as H3-28 and H3-29 to C-5 (Fig. 3) and H-6 to C-4. According to the HMBC correlations from H3-19 to C-8, C-10, and C-11, from H3-30 to C-8, C-13, and C-15, and from H3-18 to C-12, C-14, and C-17, rings B–D were deduced. Based on the HMBC correlations from H-16 to C-20, from H3-21 and 20-OH to C-17 and C-22, from H-23 to C-25, from H-24 to C-22, and from H3-26 and H3-27 to C-24, the side chain was confirmed and was located at C-17 of ring D. The acetyl group was located at C-25 (δC 79.4) by comparing the chemical shift of C-25 in 25-OH analogs (δC is approximately 71 ppm) and 25-OAc analogs (δC is approximately 79 ppm) [9]. Thus, the planar structure of 1 was determined as shown in Fig. 3.
The relative configuration of 1 was deduced by ROESY correlations (Fig. 3). Correlations of H-1β/H3-19, H-7β/H3-19, H-8/H3-18, H-8/H3-19, H-12β/H3-18, H-15β/H3-18, and H-16/H3-18 indicated that these protons should be β-oriented, while correlations of H-10/H3-30, H-12α/H3-30, H-17/H3-30, H-15α/16-OH, and H-17/16-OH showed that these protons should be α-oriented. The configuration of C-20 could not be determined by the ROESY spectrum. Accordingly, the ECD spectra of (8S,9R,10R,13R,14S,16R,17R,20R)-1 and (8S,9R,10R,13R,14S,16R,17R,20S)-1 were calculated (Fig. 4). The calculated ECD spectrum of (8S,9R,10R,13R,14S,16R,17R,20R)-1 was similar to the experimental ECD spectrum of 1. Thus, the absolute configuration of compound 1 was elucidated to be 8S,9R,10R,13R,14S,16R,17R,20R, named aquilarolide A.
The known compounds were identified as 23,24-dihydrocucurbitacin E (2) [10], cucurbitacin E (3) [11], cucurbitacin B (4) [10], (−)-(2S)-5,4ʹ-dihydroxy-7-methoxyflavanone (5) [12], and 7-hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one (6) [13] by comparison of the obtained spectroscopic data with those published in the literature.
2.3 Cytotoxic results of isolates 1–6
Isolates 1–6 were evaluated for their cytotoxicities against SPC-A-1, NCI-H520, A549, A549/Taxol, and BEAS-2B cell lines (Table 5). Aquilarolide A (1), cucurbitacin E (3), cucurbitacin B (4), and 7-hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one (6) displayed observable cytotoxicity against four tested cancer cell lines with IC50 values ranging from 0.001 to 1.84 μM and against the normal BEAS-2B cell line with IC50 values ranging from 3.46 to > 40 μM. All four active compounds, with activity strengths of 3 (IC50 = 0.002 μM) > 4 (IC50 = 0.007 μg/mL) > 1 (IC50 = 0.20 μM) > 6 (IC50 = 0.91 μM), had better inhibitory activities against A549/Taxol cells than paclitaxel (IC50 = 1.80 μM) (Table 5).
These active compounds belong to cucurbitane-type triterpenoids (1, 3, and 4) and a 2-(2-phenylethyl)chromone (6). This result agrees with those reported in the literature that cucurbitane-type triterpenoids were found to be the main constituents contributing to the cytotoxic activities in A. sinensis fruits and peels [14, 15].
Both cucurbotacin E (3) and 23,24-dihydrocucurbitacin E (2) have a four-ringed core structure in the cucurbitane skeleton, except for the side chain with an olefinic bond at C-23 in compound 3. Cucurbitacin E showed significant cytotoxic activities against human lung cancer SPC-A-1, NCI-H520, A549, and A549/Taxol cell lines with IC50 values less than or equal to 0.02 μM. However, 23,24-dihydrocucurbitacin E (2) was inactive. This indicated that the side chain with the olefinic bond at C-23 seems to be key to the cytotoxic activity of this type of compound.
The inhibitory activities of cucurbitacins E (3) and B (4) were close to each other, with IC50 values less than or equal to 0.03 μM. However, the inhibitory activity of 1 was significantly weaker than that of compounds 3 and 4. The difference between 1 and 3 and 4 is ring A. This indicates that the structure of ring A is also key to the cytotoxic activity of this type of compound. The cytotoxic potency of cucurbitacins in A549 cells was related to multivariate factors, among which the electrophilicity of molecules played a pivotal role, according to the multivariate structure–activity relationship (SAR) and quantitative structure–activity relationship modeling (QSAQ) analyses of cucurbitacin derivatives [16].
3 Experimental section
3.1 General experimental procedures
The reagents and instrumentation utilized for extraction, isolation, and structure characterization throughout this study are described in Additional file 1.
3.2 Collection of plant samples
The flowers of Aquilaria sinensis were collected from Menghai County, Yunnan Province, China, in 2019. A voucher specimen (No. KIB001-003) was identified by Ms. Jun Yang at Kunming Institute of Botany, Chinese Academy of Sciences.
3.3 Preparation of extractions and fractions and isolation of compounds
Air-dried, powdered flowers (50.0 g) of A. sinensis were extracted under ultrasound with H2O (500 mL × 3) at 60 ℃ for 30 min. The remaining residue was further extracted with 90% EtOH (500 mL × 3) at 60 ℃ for 30 min and the solvent was removed to yield crude extract PXS65 (2.4 g).
Air-dried, powdered flowers (1.2 kg) of A. sinensis were extracted under ultrasound with 90% EtOH (2 L × 4) at 60 ℃ for 30 min and the solvent was removed to yield crude extract PXS66 (174.2 g). PXS66 was suspended in water (500 mL) and then partitioned in turn with petroleum ether (500 mL × 4), EtOAc (500 mL × 4), and n-BuOH (500 mL × 4) to yield three fractions, PXS66-1 (21.1 g), PXS66-2 (23.0 g), and PXS66-3 (54.0 g), respectively. The solvent in the remaining water phase was removed to yield PXS66-4 (54.9 g).
PXS66-2 (23.0 g) was subjected to column chromatography (silica gel; CH2Cl2/MeOH, 1:0 → 0:1, v/v) to yield six further fractions (B-1–B-6). Fr. B-1 was separated on an RP C18 silica gel column eluted with MeOH/H2O (5% → 100%) to yield twelve further fractions (B-1-1–B-1-12). The 50% MeOH-eluted portion (Fr. B-1–6) was purified by column chromatography (silica gel; petroleum ether/EtOAc, 5:1 → 0:1, v/v) to yield six further fractions (B-1-6-1–B-1-6-6). Fr. B-1-6-1 was recrystallized from MeOH to yield 5 (92.2 mg). Fr. B-1-6-3 was purified by Sephadex LH-20 column chromatography (MeOH) and recrystallized from MeOH to yield 2 (11.0 mg). Fr. B-1-6-4 was recrystallized from MeOH to yield 3 (8.9 mg), and the remaining mother liquor was subjected to Sephadex LH-20 column chromatography (MeOH) to yield four further fractions (B-1-6-4-1–B-1-6-4-4). Fr. B-1-6-4-1 (33.5 mg) was purified by semipreparative high-performance liquid chromatography (HPLC) (Welch Ultimate AQ-C18, 7.8 × 250 mm, MeOH/H2O, 20:70, v = 2 mL/min) to yield 1 (3.1 mg, tR = 24.543 min) and 4 (16.6 mg, tR = 28.464 min). Fr. B-1–6-4–2 (72.6 mg) was purified by semipreparative HPLC (Welch Ultimate AQ-C18, 7.8 × 250 mm, MeOH/H2O, 15:85, v = 2 mL/min) to yield 6 (2.0 mg, tR = 27.467 min). The 60% MeOH-eluted portion (Fr. B-1–7) was purified by column chromatography (silica gel; petroleum ether/EtOAc, 5:1 → 0:1, v/v) to yield six further fractions (B-1-7-1–B-1-7-6). Fr. B-1-7-5 and Fr. B-1–7-6 were recrystallized from MeOH to yield 3 (18.5 mg).
Aquilarolide A (1). White amorphous powder; [α]D24 − 7.1 (c 0.10, MeOH); ECD (c 0.056, MeOH) λmax (Δε) 334 (− 0.22), 298 (+ 2.68), 219 (− 1.67), 199 (+ 5.67) nm; UV (MeOH) λmax 282 (2.70), 229 (3.84) nm; 1H and 13C NMR data, see Table 6; ESI–MS m/z 567 [M + Na]+; HRESIMS m/z 567.2937 [M + Na]+ (calcd. for C31H44NaO8, 567.2934) (Additional file 1).
3.4 MTS assay for cytotoxicity
The cytotoxicity activities were evaluated by MTS assay as previously described [17].
3.5 Computational methods
The absolute configuration of the new compound was determined by time-dependent density functional theory (TDDFT) calculations of ECD spectra according to our previously published paper [18].
4 Conclusion
In this study, bioassay-guided fractionation and purification were used to isolate the cytotoxic compounds of the extract from A. sinensis flowers. First, the crude extract showed significant inhibitory activities against 16 cancer cell lines with the most significant activities against the lung cancer SPC-A-1, NCI-H520, and A549 cell lines. Second, all fractions, subfractions, and pure compounds were screened for their cytotoxic activity against lung cancer SPC-A-1, NCI-H520, A549, and A549/Taxol cell lines and normal human bronchial epithelial BEAS-2B cells. From the active fraction, six compounds, including a new cucurbitane-type triterpenoid, aquilarolide A (1), five known compounds, namely, 23,24-dihydrocucurbitacin E (2), cucurbitacin E (3), cucurbitacin B (4), (−)-(2S)-5,4ʹ-dihydroxy-7-methoxyflavanone (5), and 7-hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one (6), were identified. Compounds 1, 3, 4, and 6 showed significant cytotoxicity activities against these four human lung cancer cell lines. All four active compounds, with activity strengths of 3 > 4 > 1 > 6, had better inhibitory activities against A549/Taxol cells than paclitaxel. Further studies are needed to evaluate in vivo antitumor activities and clarify the mechanisms of these active compounds.
References
El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents Med Chem. 2021;21:149–61.
Du J, Li J, Gao M, Guan Q, Liu T, Wu Y, Li Z, Zuo D, Zhang W, Wu Y. MAY, a novel tubulin inhibitor, induces cell apoptosis in A549 and A549/Taxol cells and inhibits epithelial-mesenchymal transition in A549/Taxol cells. Chem Biol Interact. 2020;323:109074.
Wang Y, Gilbert MG, Mathew B, Brickell CD, Nevling LI. Thymelaeaceae. In: Wu Z-Y, Raven PH, Hong D-Y, editors. Flora of China, vol. 13. Beijing: Science Press & Missouri Botanical Garden Press; 2007. p. 213–50.
Li W, Chen H-Q, Wang H, Mei W-L, Dai H-F. Natural products in agarwood and Aquilaria plants: chemistry, biological activities and biosynthesis. Nat Prod Rep. 2021;38:528–65.
Mei W-L, Lin F, Dai H-F. GC-MS analysis of volatile constituents from flowers and fruits of Aquilaria sinensis. J Trop Subtrop Bot. 2009;17:305–8.
Chu C-W, Li W-J, Li H-T, Huang J-C, Chung M-I, Chen C-Y. Flavonoids from the flowers of Aquilaria sinensis. Chem Nat Compd. 2016;52:497–8.
Yuan H, Zhao J, Wang M, Khan SI, Zhai C, Xu Q, Huang J, Peng C, Xiong G, Wang W. Benzophenone glycosides from the flower buds of Aquilaria sinensis. Fitoterapia. 2017;121:170–4.
Zhang X, Li H, Wang W, Chen T, Xuan L. Lipid-lowering activities of cucurbitacins isolated from Trichosanthes cucumeroides and their synthetic derivatives. J Nat Prod. 2020;83(12):3536–44.
Jacobs H, Singh T, Reynolds WF, McLean S. Isolation and 13C-NMR assignments of cucurbitacins from Cayaponia angustiloba, Cayaponia racemosa, and Gurania subumbellata. J Nat Prod. 1990;53:1600–5.
Ryu SY, Lee SH, Choi SU, Lee CO, No Z, Ahn JW. Antitumor activity of Trichosanthes kirilowii. Arch Pharm Res. 1994;17:348–53.
Maatooq G, El-Sharkawy S, Afifi MS, Rosazza JPN. Microbial transformation of cucurbitacin E 2-O-β-D-glucopyranoside. J Nat Prod. 1995;58:165–71.
Valdés E, González C, Díaz K, Vásquez-Martínez Y, Mascayano C, Torrent C, Cabezas F, Mejias S, Montoya M, Martín C-S, Muñoz MA, Joseph-Nathan P, Osorio M, Taborga L. Biological properties and absolute configuration of flavanones from Calceolaria thyrsiflora Graham. Front Pharmacol. 2020;11:1125.
Wu B, Kwon SW, Hwang GS, Park JH. Eight new 2-(2-phenylethyl)chromone (= 2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv Chim Acta. 2012;95:1657–65.
Mei W-L, Lin F, Zuo W-J, Wang H, Dai H-F. Cucurbitacins from fruits of Aquilaria sinensis. Chin J Nat Med. 2012;10:234–7.
Zhang X, Tao M-H, Chen Y-C, Gao X-X, Tan Y-Z, Zhang W-M. Five cucurbitacins from Aquilaria sinensis peels and their cytotoxic activities. Nat Prod Res Dev. 2014;26:354–7.
Silva IT, Carvalho A, Lang KL, Dudek SE, Masemann D, Duran FJ, Caro MSB, Rapp UR, Wixler V, Schenkel EP, Simões CMO, Ludwig S. In vitro and in vivo antitumor activity of a novel semisynthetic derivative of cucurbitacin B. PLoS ONE. 2015;10:e0117794.
Yang J, Su Y, Luo J-F, Gu W, Niu H-M, Li Y, Wang Y-H, Long C-L. New amide alkaloids from Piper longum fruits. Nat Prod Bioprospect. 2013;3:277–81.
Wei S-Y, Hu D-B, Xia M-Y, Luo J-F, Yan H, Yang J-H, Wang Y-S, Wang Y-H. Sesquiterpenoids and 2-(2-phenylethyl)chromone derivatives from the resinous heartwood of Aquilaria sinensis. Nat Prod Bioprosp. 2021;11:545–55.
Acknowledgements
This study was supported by Beijing Sino-Science Aquilaria Technology Co., Ltd., Beijing, China (Grant No. KET202101).
Funding
Funding was provided by Beijing Sino-Science Aquilaria Technology Co., Ltd., Beijing, China (Grant No. KET202101).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicts of interest associated with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
General experimental procedures, computational methods for the ECD of compound 1, and NMR, HRESIMS, and ECD spectra of compound 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, J., Hu, DB., Xia, MY. et al. Bioassay-guided isolation of cytotoxic constituents from the flowers of Aquilaria sinensis. Nat. Prod. Bioprospect. 12, 11 (2022). https://doi.org/10.1007/s13659-022-00334-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-022-00334-3