Abstract
One previously undescribed angeloylated noreudesmane sesquiterpenoid, dobinin O (1), along with four known eudesmane sesquiterpenoids (2–5) were isolated from the peeled roots of Dobinea delavayi. Their structures were elucidated by extensive spectroscopic data analyses. In addition, compound 1 exhibited moderate antimalarial activity against Plasmodium yoelii BY265RFP with the inhibition ratio of 17.8 ± 13.3% at the dose of 30 mg/kg/day.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dobinea delavayi (Baill.) Baill. is a perennial herb with purple-brown, cylindrical and bulky roots, which is distributed in Yunnan and Sichuan provinces of China at the altitude from 1100 to 2300 m [1]. The shape of processed roots of D. delavyi likes a sheep’s horn. Therefore, D. delavyi is called “Yang-Jiao-Tian-Ma” in China, and used to treat cough due to heat in the lung, traumatic injury, mumps, mastitis, sores, furuncles, and so on, in the folk [2].
In our previous study, D. delavayi showed significant antimalarial activity against Plasmodium yoelii BY265RFP. Subsequently, five angeloylated eudesmane sesquiterpenoid dimers dodelates A–E, with moderate antimalarial activities, were isolated from the roots of D. delavayi [3]. In our ongoing study, one previously undescribed angeloylated noreudesmane sesquiterpenoid, dobinin O (1), along with four known eudesmane sesquiterpenoids (2–5) were isolated from the peeled roots of Dobinea delavayi. Their structures were elucidated by extensive spectroscopic data analyses. In addition, compound 1 exhibited moderate antimalarial activity against Plasmodium yoelii BY265RFP with the inhibition ratio of 17.8 ± 13.3% at the dose of 30 mg/kg/day.
2 Results and Discussion
Compound 1 was obtained as white powder, and assigned the molecular formula C19H28O5 (six degrees of unsaturation) from its HRESIMS and 1H and 13C NMR spectra (including DEPT). The 1H NMR data of 1 (Table 1; Fig. S1 in Supporting Information) showed one enolic hydroxyl at δH 15.96 (s), five methyls at δH 0.96, 1.33, 1.89, 1.98 and 2.17, one olefinic proton at δH 6.09 (1H, qq, J = 7.2, 1.5 Hz), and two protons from oxygenated methines or hydroxyls at δH 4.76 (1H, dd, J = 11.6, 4.3 Hz) and 3.80 (1H, s). Another nine proton resonances from either methines or methylenes were found to occur in a relatively high-field region (between δH 1.47 and 2.74). Analyses of the 13C NMR spectrum of 1 with the aid of the DEPT-90 and -135 spectra revealed the existence of 19 carbon resonances (Table 1; Fig. S2 in Supporting Information), including five methyls (δC 15.9, 18.0, 19.2, 20.8 and 25.3), four sp3 methylenes, two sp3 methines (including one oxy-methine at δC 81.6), one sp2 methine (δC 137.9), two sp3 quaternary carbons (δC 34.0 and 73.8), and five sp2 quaternary carbons (δC 106.5, 129.3, 167.7, 180.4 and 200.4). Among them, an angeloyl was recognized easily by comparing its chemical shifts with those of reported compounds [4].
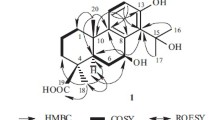
Analysis of 1H–1H COSY spectrum of 1 (Fig. 2) gave three partial structures: H2-1/H2-2/H-3, H-5/H2-6, and H-3′/H3-4′. The Me-14 (δH 0.96, s) showed HMBC correlations (Fig. 2) to C-1 (δC 38.7), C-5 (δC 50.0) and C-9 (δC 50.0), which suggested Me-14 bonded to C-10. Similarly, HMBC correlations from Me-15 (δH 1.33, s) to C-3 (δC 81.6) and C-5 suggested Me-15 jointed to C-4. HMBC correlations from the enolic hydroxyl (δH 15.96) to C-7 (δC 106.5) and C-9 suggested it jointed to C-8. Associate with HMBC correlations of H2-6 (δH 2.72, dd, J = 15.3, 4.6 Hz and δH 2.35, overlap) with C-8 (δC 180.4) and C-11 (δC 200.4), H2-9 (δH 2.32 and 2.06, both overlap) to C-7, and Me-12 (δH 2.17) only to C-7 and C-11, a noreudesmane sesquiterpenoid moiety in 1 was established. In addition, the angeloyl could be positioned at C-3 in compound 1, owing to the HMBC correlation of H-3 (δH 4.76) to C-1′ (δC 167.7). The relative configuration of 1 was characterized by interpretation of the ROESY spectrum (Fig. 2). The observation of ROESY correlations of H-3 with H-5 (δH 1.72, dd, J = 12.4, 4.6 Hz), and Me-14 to Me-15, indicated that H-3 and H-5 were in the same orientation, and Me-14 and Me-15 were in the opposite direction against H-3 and H-5. Based on biosynthetic grounds, the absolute configuration of 1 was suggested as dodelates A–E [3] and coexisting compounds 2–5. Therefore, compound 1 was finally assigned as shown in Fig. 1, and named dobinin O. A hypothetical biosynthetic pathway for compound 1 was proposed as shown in Scheme 1.
By comparison of their spectroscopic data and physicochemical properties with those reported in the literature, the four known eudesmane sesquiterpenoids were identified as dobinin A (2) [4], 3β-angeloyloxy-4α,8β-dihydroxy-eudesm-7(11)-en-8α,12-olide (3) [5], dobinin C (4) [4], and furanoeudesmane B (5) [6], respectively (Fig. 2).
Compound 1 was evaluated for its in vivo antimalarial activity against Plasmodium yoelii BY265RFP in mice, according to a four-day suppressive test [3]. As shown in Table 2, compound 1 exhibited moderate antimalarial activity with the inhibition ratio of 17.8 ± 13.3% at the dose of 30 mg/kg/day. This was further confirmed by the features of relief of hepatomegaly, increase of number of erythrocyte and content of hemoglobin, and recovery of abdominal temperature when the infected mice were treated with compound 1 (Tables S1–S3, Supporting Information). Furthermore, the immunomodulatory effect of compound 1 on the host response was also evaluated by the levels of splenic CD4+CD25+ regulatory T cells (Tregs), IL-10, IL-12, IFN-γ and IgG. As shown in Tables S4 and S5 (in Electronic supplementary material), IL-12, IFN-γ and immunosuppressive CD4+CD25+ Tregs [7, 8] from spleen significantly increased or decreased upon 1 administration at 30 mg/kg/day like those of administration of chloroquine diphosphate, suggesting that 1 could induce apoptosis of parasitized erythrocytes by elevating the level of IL-12 [9, 10], and the immunity of the infected mice could be recovered by 1 treatment.
3 Experimental Section
3.1 General
NMR spectra (1D and 2D NMR) were recorded on a Bruker Avance III-400 instrument (Bruker, Faellanden, Switzerland) with TMS as an internal reference. HRESIMS data were obtained on a Dionex Ultimate 3000 LC System (Thermo Fisher Scientific, Sunnyvale, USA) coupled in series to a Bruker Compact quadrupole time-of-flight (QTOF) mass spectrometer (Bruker, Bremen, Germany). Optical rotations were determined on a SGW-3 automatic polarimeter (Shanghai INESA Physico optiacal instrument Co., Ltd, Shanghai, P. R. China). UV data were obtained on a TU-1901 UV/Vis spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, P. R. China). IR spectra were recorded by a Nicolet 380 FT-IR spectrophotometer (Thermo Scientific, Madison, WI, USA) with KBr pellets. Silica gel (Qingdao Marine Chemical Ltd., Qingdao, P. R. China) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden) were used for open column chromatography.
3.2 Plant Material
Dried and peeled roots of Dobinea delavayi (Baill.) Baill. were purchased from the herbal medicine market of Eryuan county, Dali, Yunnan Province, P. R. China in October 2017. The material was identified by Dr. Bei Jiang, a professor from Dali University, P. R. China. A voucher specimen (No. 20171008-1) has been deposited at the Institute of Materia Medica, Dali University.
3.3 Extraction and Isolation
The roots of D. delavayi (1.1 kg) was extracted with 80% ethanol at room temperature (5 × 10 L, each for 24 h), and the extract solutions were combined and concentrated under reduced pressure. Subsequently, the resulting residue (180 g) was suspended in water and partitioned with ethyl acetate. The EtOAc soluble portion (39 g) was fractionated by a silica gel column chromatography (CC) eluting with a gradient solvent system of petroleum ether (PE)/acetone (50:1 to 0:1) to give seven major fractions (Fr. A–Fr. G). Fr. A (15 g) was subsequently separated on a silica gel CC (PE/acetone, 50:1) to give twelve subfractions Fr. A-1 to Fr. A-12. Fr. A-3 emerged as some colorless crystals after several hours settling at room temperature, then repeatedly washed with methanol to yield compound 5 (20 mg). The residual solution of Fr. A-3 was subjected to repeated Sephadex LH-20 CC (acetone) to afford compound 1 (52 mg). Fr. C (3.4 g) was separated on a silica gel CC with PE/acetone (10:1), and then was further purified by a Sephadex LH-20 CC (acetone) to give compound 2 (25 mg). Fr. D (3.2 g) emerged as some colorless crystals after several hours settling at room temperature, then repeatedly washed with methanol to yield compound 3 (28 mg). The residual solution of Fr. D was separated by CC on silica gel (PE/EtOAc, 30:1) and Sephadex LH-20 (acetone) successively, to give compound 4 (8 mg).
Dobinin O (1): white powder; \(\alpha_{{\text{D}}}^{25}\) − 41.4 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 208.0 (3.72), 288.0 (3.40) nm; IR (KBr) νmax 3463, 2935, 1716, 1612, 1366, 1247, 1160, 1080, 671 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 335.1866 [M‒H]− (calcd for C19H27O5, 335.1864).
3.4 Antimalarial Activity
The antimalarial assay was performed according to a four-day suppressive test as we previously described [3].
References
Editorial Committee of the Flora of China of Chinese Academy of Science, Flora of China, vol 45(1) (Science Press, Beijing, 1980), pp. 132–135
Editorial Board of China Herbal, State Administration of Traditional Chinese Medicine, China Herbal, vol. 13 (Scientific and Technical Publishers, Shanghai, 1999), pp. 76–677
Y. Shen, H. Chen, L. Shen, H.X. Li, X. Dong, C.J. Xiao, B. Jiang, Bioorg. Chem. 95, 103488 (2020)
Z.Q. Cheng, D. Yang, Q.Y. Ma, H.F. Dai, S.Z. Huang, X.H. Yi, J. Zhou, Y.X. Zhao, Planta Med. 78, 1878–1880 (2012)
G.M.S.P. Guilhon, A.H. Müller, Phytochemistry 49, 1347–1351 (1998)
Q. Wang, C. Ma, J. Zhai, Acta Crystallogr. C 56, e569 (2000)
P. Zhou, J. Xu, M. Dai, Y. Shi, G. Wu, Y. Fang, X. Yan, J. Viral, Hepatitis 25, 733–741 (2018)
E.M. Shevach, Nat. Rev. Immunol. 2, 389–400 (2002)
A. Ssemaganda, A.K. Giddam, M. Zaman, M. Skwarczynski, I. Toth, D.I. Stanisic, M.F. Good, Front. Immunol. 10, 135 (2019)
P. Perlmann, M. Troye-Blomberg, Malaria Immunology, 2nd edn. (Karger Publishers, Switzerland, 2002), pp. 204–228
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 81960637 and 81460532), and the Innovation Team Project of Dali University for the Development and Utilization of Characteristic Medicinal Plants in Western Yunnan & Bai Nationality Medicines (No. ZKLX2019106).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Dedicated to Professor Han-Dong Sun on the occasion of his 80th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, XR., Shen, Y., Cui, SJ. et al. A New Antimalarial Noreudesmane Sesquiterpenoid from Dobinea delavayi. Nat. Prod. Bioprospect. 10, 101–104 (2020). https://doi.org/10.1007/s13659-020-00234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-020-00234-4