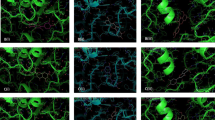
Abstract
Secondary metabolites from medicinal plants have been reported to inhibit the formation of glucose-induced protein aggregation. This study investigated the inhibitory potential of Vernonia amygdalina leaf extracts on amyloid protein aggregation. Prevention of glycation-induced amyloid-beta fibril formation was evaluated through the assessment of anti-glycation capacities of the extracts, inhibitions of glycation-induced oxidation of protein thiol groups and protein aggregation. Colorimetric methods were used for the estimation of aqueous and methanol extract of V. amygdalina. Luteolin, luteolin-7-O-β-glucuronoside, and luteolin-7-O-β-glucoside were identified through a previous study and evaluated for their binding energies with amyloid-beta and amyloid-beta fibril. The results showed that aqueous extract had higher amounts of phenolics while methanol extract had higher amounts of flavonoids contents. The aqueous extract exhibited higher albumin glycation inhibitory potential with the IC50 value of 65 µg/ml. The methanol extract possessed higher protective effects on the total thiol groups of the glycated albumin and also exhibited higher inhibition of aggregates formation when compared with the aqueous extract. Luteolin-7-O-β-glucuronoside had the highest binding affinity with the amyloid beta-peptide (1-42) and amyloid-beta (1-42) fibrils with the mean values of − 5.63 kcal/mol and −6.48 kcal/mol respectively. However, it was observed that luteolin exhibited the highest binding free energy of − 20.43 kcal/mol and − 8.67 kcal/mol with both proteins respectively. The ADMET properties revealed all the phytochemicals possess drug-like properties. These results demonstrated that phytoconstituents of V. amygdalina could act as highly potent inhibitors of protein aggregation and could be a promising candidate for the management of hyperglycaemia-induced protein aggregation.











Similar content being viewed by others
Availability of data and materials
The dataset supporting the conclusions of this article is included as tables and figures in the text.
References
Adeoye AO, Bewaji CO (2018) Chemopreventive and remediation effect of Adansonia digitata L. Baobab (Bombacaceae) stem bark extracts in mouse model malaria. J Ethnopharmacol 210:31–38
Alara OR, Abdurahman NH, Ukaegbu CI, Kabbashi NA (2019) Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. J Taibah Univ Sci 13(1):414–422
Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464
Chen G, Xu T, Yan Y, Zhou Y, Jiang Y, Melcher K, Xu HE (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica 38:1205–1235
Cole GM, Teter B, Frautschy SA (2007) Neuroprotective effects of curcumin. Adv Exp Med Bio 595:197–212
Crespi GA, Hermans SJ, Parker MW, Miles LA (2015) Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci Rep 5:9649
de la Torre JC (2002) Vascular basis of Alzheimer’s pathogenesis. Ann N Y Acad Sci 977:196–215
Duyckaerts C, Potier MC, Delatour B (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol 115:5–38
Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Dröse S, Brandt U, Fändrich M, Müller WE, Götz J (2008) Oligomeric and fibrillar species of β-amyloid (Aβ42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med 86:1255–1267
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
El-Mesallamy HO, Hamdy NM, Ezzat OA, Reda AM (2011) Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Investig Med 59:1233–1238
Feng Y, Wang XP, Yang SG, Wang YJ, Zhang X, Du XT, Sun XX, Zhao M, Huang L, Liu RT (2009) Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 30(6):986–995
Furth AJ (1988) Methods for assaying non-enzymatic glycosylation. Anal Biochem 175:347–360
Galati G, O’Brien PJ (2004) Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 37(3):287–303
Hao GF, Zhu XL, Ji FQ (2009) Understanding the mechanism of drug resistance due to a codon deletion in protoporphyrinogen oxidase through computational modeling. J Phys Chem B 113:4865–4875
Hartl FU (2017) Protein misfolding diseases. Annu Rev Biochem 86:21–26
Hou T, Wang J, Li Y (2011) Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J Chem Inf Model 51:69–82
Iakovleva I, Begum A, Pokrzywa M, Walfridsson M, Sauer-Eriksson AE, Olofsson A (2015) The flavonoid luteolin, but Not Luteolin-7-O-glucoside, prevents a transthyretin mediated toxic response. PLoS ONE 10(5):e0128222
Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso M, Fasanmade AA (1994) Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem 42(11):2445–2448
Kasahara K, Fukuda I, Nakamura H (2014) A novel approach of dynamic cross correlation analysis on molecular dynamics simulations and its application to Ets1 dimer–DNA complex. PLoS ONE 9:e112419
Klunk WE, Jacob RF, Mason RP (1999) Quantifying amyloid by Congo red spectral shift assay. Methods Enzymol 309:285–305
Kuhnle G, Spencer JP, Chowrimootoo G, Schroeter H, Debnam ES, Srai SK, Rice-Evans C, Hahn U (2000) Resveratrol is absorbed in the small intestine as resver-atrol glucuronide. Biochem Biophys Res Commun 272:212–217
Lee SJ, Lim HS, Masliah E, Lee HJ (2011) Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res 70:339–348
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46(1–3):3–26
Liu R, Lan X, Ying J, Du GH (2010) Protective effects of luteolin against amyloid β25–35-induced toxicity on rat cerebral microvascular endothelial cells. Chin J Nat Med 8(3):223–227
Nwanjo HU (2005) Efficacy of aqueous leaf extract of Vernonia amygdalina on plasma lipoprotein and oxidative status in diabetic rat models. Nig J Physiol Sci 20(1–2):39–42
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3(10):33
Olasehinde TA, Olaniran AO, Okoh AI (2016) Phenolic composition and evaluation of methanol and aqueous extracts of bitter gourd (Momordica charantia L.) leaves on angiotensin-I-converting enzyme and some pro-oxidant induced lipid peroxidation in vitro. J Evid-Based Comp Alt Med 21(4):67–76
Oso BJ, Olaoye IF (2020) Comparative in vitro studies of antiglycemic potentials and molecular docking of Ageratum conyzoides L. and Phyllanthus amarus L. methanolic extracts. SN App Sci 2:629
Oso BJ, Oyeleke O, Soetan O (2018) Influence of different solvent polarities on the phenolics, flavonoids and antioxidant properties of the fruit of Xylopia aethiopica (Dunal) A. Rich. Trends Phytochem Res 2(2):97–102
Pan Y, Gao D, Zhan CG (2008) Modeling the catalysis of anti-cocaine catalytic antibody: competing reaction pathways and free energy barriers. J Am Chem Soc 130:5140–5149
Parveen A, Kim JH, Oh BG, Subedi L, Khan Z, Kim SY (2018) Phytochemicals: target-based therapeutic strategies for diabetic retinopathy. Molecules 23(7):1519
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201
Pires DE, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graphbased signatures. J Medicinal Chem 58(9):4066–4072
Raha K, Merz KM (2005) Large-scale validation of a quantum mechanics based scoring function: predicting the binding affinity and the binding mode of a diverse set of protein-ligand complexes. J Med Chem 48:4558–4575
Safari M, Sheikh N, Kashani KM (2010) Study on the effect of vitamin C on the in vitro albumin glycation reaction. Iran J Pharm Res 5(4):275–279
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Soto C, Estrada LD (2008) Protein misfolding and neurodegeneration. Arch Neurol 65(2):184–189
Sousa MC, Braga RC, Cintra BA, de Oliveira V, Andrade CH (2013) In silico metabolism studies of dietary flavonoids by CYP1A2 and CYP2C9. Food Res Int 50(1):102–110
Trivella DB, dos Reis CV, Lima LM, Foguel D, Polikarpov I (2012) Flavonoid interactions with human transthyretin: combined structural and thermodynamic analysis. J Struct Biol 180(1):143–153
Trott O, Olson AJ (2010) AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem 31(2):455–461
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93
Wang W, Tan M, Yu J, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136
Yang JF, Wang F, Chen YZ, Hao GF, Yang GF (2019) LARMD: integration of bioinformatic resources to profile ligand-driven protein dynamics with a case on the activation of estrogen receptor. Brief Bioinform. https://doi.org/10.1093/bib/bbz141
Zhang JX, Xing JG, Wang LL, Jiang HL, Guo SL, Liu R (2017) Luteolin inhibits fibrillary β-Amyloid1–40-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules 22(3):334
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoids contents in mulberry and their scavenging effects on super oxides radicals. Food Chem 64(4):555–559
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AOA and BJO conceived, designed and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Akinwunmi O. Adeoye has no conflict of interest. Babatunde Joseph Oso has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adeoye, A.O., Oso, B.J. Investigative studies on the inhibition of amyloid-like fibrils formation by the extracts of Vernonia amygdalina Del. leaf. ADV TRADIT MED (ADTM) 22, 163–176 (2022). https://doi.org/10.1007/s13596-020-00535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-020-00535-6