Abstract
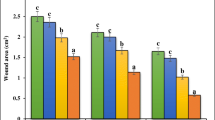
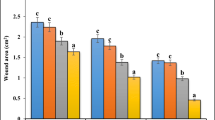
The present study was to evaluate in vivo cutaneous wound healing activity of hydromethanolic extract of M. jalapa radix and to validate its traditional use as medicine for wound healing. In excision wound model, the wound area was measured on the day of infliction (zero day) and subsequently at 4 days interval until the completion of wound healing. In dead space wound model, on 10th post wounding day, the granulation tissues were harvested for estimation of blotting of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF 2) and collagen type III (COL 3A), hydroxyproline content and histological studies. A significant increase (p < 0.05) in rate of wound contraction, decrease in Colony Forming Unit (CFU) count and decrease in epithelisation period was observed in 5 and 10 % treated groups as compared to control. A significant increase (p < 0.05) in hydroxyproline content, up- regulated expression of COL 3A was observed in treated groups as compared to control which was supported by histological evidences. Increase in rate of wound contraction, hydroxyproline content and COL 3A, decrease in epithelisation period and CFUs count shows the wound healing activity of M.jalapa and validate its traditional use in wound and inflammation.





Similar content being viewed by others
References
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burnette WN (1981) “Western blotting” : electrophoretic transfer of proteins from sodium dodecyl sulphate−polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Copenhaver WM, Bunge RP, Bunge MB (1967) Bailey’s textbook of histology, 16th edn. Williams &Wilkins
Cristiani IBW, Gabriela T, Mateus FR, Carina F, Maria EP, Juliano F, Melânia PM (2008) Antinociceptive activity of Mirabilis jalapa in mice. J Ethnopharmacol 120:169–175
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Evans WC (1997) Trease and Evans pharmacognosy, 14th edn. Harcourt Brace and company:Asia Pvt. Ltd. Singapore
Fatima AD, Modolo LV, Sanches ACC, Porto RR (2008) Wound healing agents: the role of natural and non-natural products in drug development. Mini Rev Med Chem 8:879–888
Ferrara N (1999) Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77:527–543
Gogoi J, Nakhuru KS, Rai AK, Chattophadhayay P, Gogoi HK, Singh L (2012) Antimicrobial and free radical scavenging activities of crude extract and fractions from the tuberous root of Mirabilis jalapa L. South Asian J Exp Biol 2:209–216
Goldfarb M (2001) Signaling by fibroblast growth factors: the inside story. doi:10.1126/stke.2001.106.pe37
Gutierrez RMP, Vargas SR (2006) Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia 77:286–289
Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Walsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K (1997) Proteolytic processing regulates receptor specificity and activity of VEGF – C. EMBO J 16:3898–3911
Kokate CK (1999) Practical Pharmacognosy, 4th edn. Vallabh Prakashan Publication, New Delhi
Kumar R, Katoch SS, Sharma S (2006) β-Adrenoceptor agonist treatment reverses denervation atrophy with augmentation of collagen proliferation in denervated mice gastrocnemius muscle. Indian J Exp Biol 44:371–376
Lobb RR, Alderman EM, Fett JW (1985) Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochem 24:4969–4973
Mace ME (1963) Histochemical localization of phenols in healthy and diseased tomato roots. Phytopathol 16:915–925
Maquart FX, Chastang F, Simeon A, Birembaut P, Gillery P, Wergrowski Y (1999) Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 9:289–296
Mohamed H, Jarraya R, Lassoued I, Masmoudi O, Damak M, Moncef N (2010) GC/MS and LC/MS analysis, antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochem 45:1486–1493
Morton JP, Melone MH (1972) Evaluation of vulnerary activityby an open wound procedure in rats. Arch Int Pharmacodyn 196:117–120
Mukherjee KL (2000) Medical Laboratory Technology. Tata McGraw Hill Ltd, New Delhi
Neumann RE, Logon MA (1950) The determination of collagen and elastin in tissues. J Biol Chem 186:549–556
Pfoze NL, Kumar Y, Myrboh B (2012) Survey and assessment of ethnomedical plants used in Senapati District of Manipur state, Northeast India. Phytopharmacology 2:285–311
Raghow R (1994) The role of extracellular matrix in postinflammatory wound healing and fibrosis. Fed Am Soc Exp Biol J 8:823–831
Siddiqui S, Siddiqui BS, Adil Q, Begum S (1990) Constituents of Mirabilis jalapa. Fitoterapia 61:471
Simpson T, Orgazaly B (1986) Useful medicinal plant, 1st edn. ELBS pp 555
Singer AJ, Clark RAF (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Stadelman WK, Digents AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176:39–47
Willey JM, Sherwood L, Woolverton CJ, Prescott LM, Harley JP, Klein DA (2002) Laboratory excerises in microbiology, 5th edn. The McGraw Hill Company
Yang SW (2000) Three new phenolic compounds from a manipulated plant cell culture of Mirabilis jalapa. J Nat Prod 64:313–317
Yoganarasimhan SN (2000) Medicinal plants of India. Cyber Media, Tamil Nadu, p 357
Acknowledgments
Authors are highly thankful to DRDO, New Delhi for the financial support and Semima Yeshim, Department of Life Sciences, Dibrugarh University, for her help in wound tissue sectioning.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gogoi, J., Nakhuru, K.S., Chattophadhayay, P. et al. In vivo evaluation of cutaneous wound healing activity of Mirabilis jalapa L radix. Orient Pharm Exp Med 14, 103–109 (2014). https://doi.org/10.1007/s13596-013-0122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-013-0122-6