Abstract
Based on screening for potential beneficial lactic acid bacteria from Coalho cheese produced in the North-East region of Brazil, eight strains belonging to Lactobacillus rhamnosus and Lactobacillus plantarum were selected. All investigated strains presented low levels of hydrophobicity. Different levels of coaggregation were observed for tested Lb. rhamnosus and Lb. plantarum with Listeria monocytogenes. All strains were able to grow in presence of 0.5% of the sodium salts of taurocholic acid (TC), taurodeoxycholic acid (TDC), and glycocholic acid (GC) and showed the ability to deconjugate only TC, TDC, and GC. Investigated Lb. rhamnosus and Lb. plantarum strains showed good survival when exposed to the conditions simulating the GIT conditions. Lb. rhamnosus and Lb. plantarum strains were tested for presence of virulence, antibiotic resistance, and biogenic amine production genes. In addition, minimum inhibititory concentration (MIC) of selected antibiotics was determined. Production of antimicrobial peptides (bacteriocins) was investigated. However, only Lb. rhamnosus EM253 produced bacteriocin at level 800 arbitrary unit (AU) mL−1 against L. monocytogenes 211. The bacteriocin remained stable at pH from 2.0 to 10.0 and after exposure at 100 °C for 120 min and in presence of surfactants and salts. Studied Lb. rhamnosus and Lb. plantarum strains showed good potential to be applied as a functional coculture/s with beneficial properties in the production of Coalho cheese.
Similar content being viewed by others
1 Introduction
A growing body of scientific evidence has demonstrated the relevance of the intestinal microbiota to human health and the beneficial role of probiotic microorganisms, which positively affect host health when administered regularly and in adequate amounts (FAO/WHO 2006). Probiotic food products represent a market trend nowadays, associated with the growing concern about health among consumers and with the increasing consciousness about the relationship between diet and well-being. Search for new probiotic strains among lactic acid bacteria (LAB) has attracted strong scientific interest and sets up an area of intense scientific activity (Vizoso Pinto et al. 2006; Vinderola et al. 2008; Salva et al. 2011). Despite the availability of commercial probiotic cultures, well studied and characterized, the search for new beneficial strains with promising health and technological properties is ongoing.
Lactobacillus spp. is of particular interest having health-related properties, in addition to a long history of use in food fermentations. Strains pertaining to several Lactobacillus species were shown to exhibit probiotic properties, particularly within the Lactobacillus casei group, as Lactobacillus paracasei and Lactobacillus rhamnosus strains. Lb. rhamnosus are typical inhabitants of the gastrointestinal tract and naturally found in cheese and other dairy products, and several strains have been used as probiotic in commercial food products (Salva et al. 2011). Similarly, Lactobacillus plantarum is another species that drives research interest as a potential source of beneficial strains. Commonly found in milk and cheese and considered a ubiquitous species, many Lb. plantarum strains were found to survive to the exposure to gastrointestinal conditions and have shown properties related to health promotion and maintenance (Zago et al. 2011).
Resistance to the conditions prevailing in the gastrointestinal tract (GIT) and good adhesion to the intestinal mucosa are important features for probiotic bacteria, as the majority of the beneficial effects occur in the intestinal environment. The production of enzymes such as bile salt hydrolases, which are related to the reduction of plasmatic cholesterol levels; β-galactosidase, involved in the metabolism of lactose; as well as of antimicrobial compound production all these features are properties of interest in the search of probiotic candidates (FAO/WHO 2006; Begley et al. 2006; Vizoso Pinto et al. 2006).
Even though many Lactobacillus spp. have a generally recognized as safe (GRAS) and a qualified presumption of safety (QPS) status, it is important to check the safety of each specific strain intended for use in a probiotic food formulation (Lavilla-Lerma et al. 2013). In addition, probiotic bacteria should survive during the food processing steps and throughout the product shelf-life, and it is also desirable that they can offer a contribution to the sensory attributes of the final product.
In this study, some properties related to probiotic potential and technological applications of Lb. rhamnosus and Lb. plantarum strains isolated from artisanal Coalho produced from raw bovine milk in the North-East region of Brazil were investigated. Coalho chese is a traditional Brazilian product, produced from raw milk, after rennet coagulation, without addition of commercial starter cultures, and mainly consumed by the regional population (Silva et al. 2012). A series of in vitro experiments were performed for the selection of probiotic candidates to be used in fermented dairy products.
2 Materials and methods
2.1 Lactic acid bacteria isolation and characterization
LAB were isolated from 12 different artisanal Coalho cheeses made from raw bovine milk, collected from different local markets in the city of Fortaleza (Ceará State, Brazil), produced in the regions of Jaguaribe (Ceará State, Brazil). Twenty-five grams of each cheese was added to 225 mL of 2% (w/v) sodium citrate (Vetec, Duque de Caxias, RJ, Brazil) sterile solution, homogenized in a Stomacher machine (Seward 400, Worthing, UK), and heated at 45 °C for 5 min. Serial dilutions were prepared in 0.1% (w/v) peptone water (Merck, Darmstadt, Germany) and plated in duplicate on M17 agar and Rogosa agar (Oxoid, Basingstoke, UK) followed by incubation for 48 h at 30 and 42 °C, respectively, under anaerobic conditions. LAB were enumerated (CFU g−1 cheese), and 15–20 colonies were randomly selected from M17 and Rogosa agar plate for each cheese at both experimental temperatures. Subsequently, the isolates were purified in MRS (Difco/BD, Franklin Lakes, NJ, USA) and were analyzed for cell morphology, Gram staining, catalase activity, and acid production. Acid production was examined by coagulation index and reduction on Litmus Milk medium (HiMedia Laboratories, Mumbai, India) at 35 °C for 7 days. Microorganisms, characterized as acid producers, Gram-positive, catalase-negative, with rod, or cocci shape, were preclassified as LAB.
2.2 Identification of Lactobacillus spp.
Thirty-four isolates, preselected as LAB with rod morphology, were pre-identified by API50CHL (BioMéureux, Marcy-l′Etoile, France) and classified as Lactobacillus spp. The following microorganisms Enterococcus faecalis ATCC 19443, Lactococcus lactis subsp. lactis NCDO 2003, Lactobacillus acidophilus ATCC 4522, Lb. plantarum ATCC 8014, Lb. rhamnosus GG, and Streptococcus thermophilus NCDO 1968 were used as controls in the identification process. Bacterial cultures were stored at −80 °C in presence of 20% glycerol.
Differentiation of the 34 Lactobacillus isolates was performed by random amplification of polymorphic DNA (RAPD) PCR. DNA was isolated according to the manufacture protocol using ZR Fungal/Bacterial DNA Kit (Zymo Research, Irvine, CA, USA). Primers OPL-1 (5′-GGC ATG ACC T-3′) and OPL-2 (5′-TGG GCG TCA A-3′) were used, and the amplification reactions were performed according to Todorov et al. (2010). The amplified products were separated by electrophoresis in 1.5% (w/v) agarose gels in presence of blue-green loading buffer (LGC, São Paulo, SP, Brazil) and 0.5× TBE buffer at 100 V for 2 h and visualized under UV light. Darwin software was employed for analysis of the RAPD-PCR profiles, and a dendogram was generated based on the Jaccard similarity coefficient and the unweighted pair group with arithmetic mean (UPGMA) method. Banding patterns were analyzed using Gel Compare, Version 4.1 (Applied Maths, Kortrijk, Belgium).
Taxonomical identification was confirmed by sequencing of the PCR-amplified 16S rRNA using the universal pair of primers 8F (5′-CAC GGA TCC AGA CTT TGA TYM TGG CTC AG-3′) and 1512R (5′-GTG AAG CTT ACG GYT AGC TTG TTA CGA CTT-3′), where Y means C + T and M means A + C (Todorov et al. 2010). In order to differentiate Lb. casei, Lb. plantarum, and Lb. rhamnosus, two additional 16–23S intergenic spacing regions were PCR-amplified and sequenced: The first region was amplified using the pair of primers 16-1A (5′-GAA TCG CTA GTA ATC G-3′) and 23-1B (5′-GGG TTC CCC CAT TCG GA-3′), which in Lactobacillus frequently amplify two fragments, being the smallest in the range of 500 to 600 bp (Tannock et al. 1999). The 16-1A/23-1B smallest amplified fragment was isolated from agarose gel using Qiaquick Gel Extraction kit (Qiagen, Venlo, Netherlands). The second 16–23S region analyzed was PCR-amplified using the pair of primers Lu-5 (5′-CTA GCG GGT GCG ACT TTG TT-3′) and Lac-2 (5′-CCT CTT CGC TCG CCG CTA CT-3′), resulting in a fragment of 400-bp average size (Song et al. 2000). All PCR reactions were done in a final volume of 25 μL, containing 0.5 unit of PlatinumTaq DNA polymerase (Life Technology, Carlsbad, CA, USA) and 5 pmol of each primer. Sequencing reactions were performed for forward and reverse strands using the DYEnamic™ET Cycle Sequencing kit (GE Healthcare, Piscataway, NJ, USA) and sequencer MegaBACE (GE Healthcare, Little Chalfont, UK). Blast tool and Lactobacillus genome reference sequences, from National Center for Biotechnology Information (NCBI) and listed by Song et al. (2000), were used for identification analyses. Additional sequence alignments were performed using CLC Main Workbench software V6.9.1 (Qiagen).
2.3 Beneficial properties
2.3.1 Resistance to simulated gastric and intestinal conditions
The resistance of the Lactobacillus spp. strains to gastric and intestinal conditions was evaluated through an in vitro model adapted from Vizoso Pinto et al. (2006). Overnight cultures of each strain were, respectively, used to inoculate MRS broth (Oxoid) at 2 × 108 CFU mL−1, and an aliquot of 1 mL was serially diluted in peptone water, pour-plated onto acidified MRS agar (pH 5.4), and incubated anaerobically (GasPack System, Oxoid) at 37 °C for 72 h to determine the bacterial concentrations (CFU mL−1) at time 0. To simulate gastric conditions, 6 mL of the cell suspension was diluted in 10 mL of an artificial gastric fluid consisting of a sterile electrolyte solution (6.2 g.L−1 NaCl, 2.2 g.L−1 KCl, 0.22 g.L−1 CaCl2, and 1.2 g.L−1 NaHCO3, pH 2.5) supplemented with 0.3% pepsin (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 1 h at 37 °C under continuous agitation (150 rpm; Dubnoff Bath, Tecnal, Piracicaba, SP, Brazil). One-milliliter aliquot was sterilely removed to determine the CFU mL−1 as described before. To simulate the passage through the small intestine, 2 mL of the remaining suspension was diluted in 8-mL artificial duodenal secretion (pH 7.2) consisting of 6.4 g.L−1 NaHCO3, 0.239 g.L−1 KCl, 1.28 g.L−1 NaCl, 0.5% bile salts (Oxgall, Merck, Darmstadt, Germany), and 0.1% pancreatin (Sigma-Aldrich). After 3 h of incubation at 37 °C under continuous agitation (150 rpm), 1-mL aliquots were removed for determination of the final CFU mL−1. The assay was performed three times for each strain, and the Lactobacillus spp. enumeration was done in duplicate. The survival rate (SR) of Lactobacillus spp. after gastric and enteric simulation were calculated according to Wang et al. (2009), using the equation: SR (%) = [log CFU N / log CFU N0] × 100, where N 0 and N are the population values before and after the assay, respectively.
2.3.2 Bile salt deconjugation
To evaluate the Lactobacillus spp. strain’s ability to perform bile salt deconjugation, overnight cultures of each isolate were streaked on MRS agar plates previously prepared containing 0.5% (w/v) of the sodium salts of taurocholic acid (TC), taurodeoxycholic acid (TDC), glycocholic acid (GC), and glycodeoxycholic acid (GDC) (Sigma-Aldrich). After anaerobic incubation (GasPack System, Oxoid) at 37 °C for 72 h, the presence of an opaque halo around colonies was considered positive for bile salt deconjugation (dos Santos et al. 2014a). The test was performed in two independent experiments in duplicate.
2.3.3 β-Galactosidase activity
The β-galactosidase activity of selected Lactobacillus spp. strains was assessed employing sterile filter paper disks impregnated with o-nitrophenyl-β-d-galactopyranose (ONPG Disks, Fluka, Buchs, Switzerland), according to the manufacturer instructions. Overnight culture of each strain was streaked on MRS agar plates and incubated anaerobically (GasPack System, Oxoid) at 37 °C for 48 h. A colony of each strain was picked up and emulsified in a tube containing ONPG disk added with 0.1 mL of sterile 0.85% (w/v) sodium chloride solution. The tubes were incubated at 35 °C and observed at an interval of 1 h, for up to 6 h. The release of a yellow chromogenic compound, o-nitrophenol, indicates a positive result for production of β-galactosidase. The test was performed in two independent experiments in duplicate.
2.3.4 Cell surface hydrophobicity
Cell surface hydrophobicity was evaluated according to dos Santos et al. (2014a). Overnight stationary-phase cultures of Lactobacillus spp. strains were centrifuged at 7,000×g for 5 min at 4 °C (Centrifuge 5810R, Eppendorf, Hamburg, Germany), washed twice, and resuspended in phosphate buffer (50 mM K2HPO4/KH2PO4, pH 6.5) to reach value around 1.0 of OD at A560 values (A0). N-hexadecane was added to the cell suspension in proportion 1:5 (N-hexadecane (Sigma-Aldrich)/cell suspension), and the mixture was homogenized for 2 min. After 1-h incubation at 37 °C, the A560 value (A) of the aqueous layer was measured. Cell surface hydrophobicity was calculated according to the equation: %H = [(A0–A) / A0] × 100, where A0 and A are the absorbance values before and after extraction with the organic solvent, respectively. The assay was performed in sextuplicate.
2.3.5 Autoaggregation and coaggregation with Listeria monocytogenes strains
For the evaluation of autoaggregation, Lactobacillus spp. strains were grown in MRS broth (Oxoid) for 24 h at 37 °C. The cells were harvested by centrifugation (7,000×g for 10 min at 20 °C), washed, resuspended, and diluted in 0.85% sterile saline to an OD660nm of 0.3 using a spectrophotometer (Ultrospec 2000 UV/Visible Spectrophotometer, Pharmacia Biotech, Cambridge, UK). After incubation at 37 °C for 60 min, OD660nm was recorded again. Autoaggregation was determined using the following equation (Todorov et al. 2008): % Autoaggregation = [(OD0–OD60) / OD0] × 100. OD0 refers to the initial OD, and OD60 refers to the OD determined after 60 min. For the determination of OD60, the cultures were centrifuged at 300×g for 2 min at 20 °C.
For evaluation of coaggregation, Lactobacillus spp. strains were grown in MRS broth and Listeria monocytogenes in BHI (Oxoid), for 24 h at 37 °C. Cells that were prepared in similar manner to cell suspensions reach an OD660nm of 0.3. Then, 750 μL of each Lactobacillus spp. suspension was mixed with 750 μL of L. monocytogenes coaggregation partner [L. monocytogenes ScottA, L. monocytogenes serotype 4b (211, 302, and 620) and L. monocytogenes serotype 1/2a (506)] for 30 s, and the OD660nm recorded at time 0 and over 60 min. Coaggregation was calculated using the following equation (Todorov et al. 2008): % Coaggregation = [(OD0–OD60) / OD0] × 100. OD0 refers to the initial OD, taken immediately after the tested strains were mixed, and OD60 refers to the OD of the supernatant after 60-min incubation at 37 °C and centrifuged at 300×g for 2 min at 20 °C. Experiments were conducted in triplicate on two independent assays.
2.3.6 Assessment of antagonism and preliminary characterization of the inhibitory metabolites
Lactobacillus spp. strains were screened for antagonism against L. monocytogenes species from different serological groups by the spot method (Todorov et al. 2010) with slight modifications. Briefly, 10-μL cell-free supernatant obtained after centrifugation (10,000×g at 4 °C for 10 min) of overnight MRS cultures, anaerobically grown to avoid the effect of H2O2, was spotted onto BHI enriched with 1.0% agar seeded with 1% (v/v) of overnight culture of the indicator strain, leading to a final concentration of around 106 CFU mL−1. The incubation was carried out aerobically at 37 °C for 18 h. The MRS medium was tested as a negative control. In addition, antimicrobial activity was screened against number of L. monocytogenes, Enterococcus spp., and various LAB. To identify the inhibitory substances secreted into the growth medium, Lactobacillus spp. strains presenting antagonism were grown overnight at 30 °C in 20-mL broth. A cell-free supernatant obtained by centrifugation (10,000×g, 10 min, 4 °C), filtered with 0.22-μm pore size Acrodisc® syringe filters (Pall Gelman Laboratory, East Hills, NY, USA), and treated for 10 min at 80 °C were separated in two samples designed as sample A, directly tested, and sample B, pH adjusted to 6.5 with 1 M NaOH. The antagonistic activities were determined at least in triplicate with the spot diffusion assay, and the presence of any inhibitory zone was recorded after 18 h of incubation at 37 °C (Todorov et al. 2010).
The cell-free supernatants obtained from the antagonistic strains were treated with proteases such as α-chymotrypsin, proteinase K, pepsin, trypsin, protease XIV and papain (Sigma-Aldrich), and catalase, lipase and α-amylase (Sigma-Aldrich) at 1.0 mg.mL−1 and incubated for 1 h at 30 °C. Each cell-free supernatant was adjusted to pH 6.5 with 1 M NaOH, filter sterilized (0.22 μm), and treated with the mentioned enzymes. The treated and untreated cell-free supernatants (control samples) were heated at 95 °C for 5 min, and then immediately cooled in ice to inactivate the enzymes. The residual activity was determined by measuring the diameter of inhibition zones.
Bacteriocin-producing strains were cultured in MRS broth at 30 °C for 24 h, and a cell-free supernatant was obtained as described above. The effect of pH, temperature, and selected chemicals on the stability of the antimicrobial substance(s) was performed by treatment of the cell-free supernatant as described by dos Santos et al. (2014b).
Dynamic of bacterial growth and bacteriocin production by selected strains were determined by culturing in MRS broth overnight at 30 °C and inoculating a flask with 200-mL MRS broth, then cultured in static condition at 30 °C for 36 h. The bacterial growth was monitored every hour based on changes in turbidity at 600 nm (Ultrospec 2000 UV/Visible Spectrophotometer). The production of bacteriocin was determined every 3 h and expressed in arbitrary unit (AU) per mL against L. monocytogenes 211 and Enterococcus faecium ATCC 19433, according to Todorov et al. (2010). Activity was expressed as AU per mL, with one AU defined as the reciprocal of the highest dilution showing a clear zone of inhibition (Todorov et al. 2010).
Adsorption of bacteriocin produced by selected strains to its own producer cells was studied according to method proposed by Yang et al. (1992).
Growth of the test microorganisms in presence of bacteriocin produced by Lb. rhamnosus EM253 was determined according to dos Santos et al. (2014b). A 20-mL aliquot of bacteriocin-containing filter-sterilized (0.20 μm, Minisart®, Sartorius) supernatant (pH 6.0) was added to 100-mL culture of L. monocytogenes 211 and E. faecium ATCC 19433 in early exponential phase (OD600nm = 0.15) and incubated for 12 h. Optical density readings (at 600 nm) were recorded at 1-h interval.
2.4 Technological characterization
2.4.1 Growth in milk and viability in acidified milk
Growth of Lactobacillus spp. strains in milk was evaluated according to Vinderola et al. (2008) with modifications. Overnight cultures were centrifuged (6,000g, 15 min, 4 °C), washed twice with phosphate buffer saline (PBS), pH 7.4, inoculated at 10% (v/v) in sterile-reconstituted skim milk (10%, w/v, Difco), and incubated at 35 °C in anaerobic conditions. Changes in milk pH were recorded after 6, 24, and 48 h of incubation.
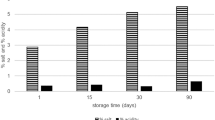
In addition, strain viability in acidified milk was investigated as described by Vinderola et al. (2008). Each cell suspension was inoculated (1.5%, v/v) in skim milk (10%, w/v, Difco) previously acidified with lactic acid to pH 4.0 and 5.0. Growth in skim milk without changes of pH served as control. Cultures were stored at 5 °C for 30 days. Cell viability was performed by determination of CFU mL−1 by plating on MRS agar at days 0 and 30 to evaluate the lactobacilli viability during cold storage.
2.4.2 Interactions with commercial starter and adjunct cultures
Antagonistic interactions between selected Lactobacillus spp. strains and commercial LAB cultures commonly used in dairy products manufacture, St. thermophilus TA-040 (Danisco), St. thermophilus, and Lb. delbrueckii subsp. bulgaricus YF-L812 (Chr. Hansen), and also Lc. lactis subsp. lactis and Lc. lactis subsp. cremoris R-704 (Chr. Hansen), were investigated by well diffusion agar assay (Vinderola et al. 2008).
2.5 Safety evaluation of selected Lactobacilli strains
2.5.1 Susceptibility to antimicrobials
Antimicrobial Susceptibility Test Discs (Oxoid) were employed to assess susceptibility of selected Lactobacillus spp. strains to antimicrobials presenting different modes of action: penicillin G (10 μg per disk), ampicillin (10 μg per disk), vancomycin (30 μg per disk), gentamicin (10 μg per disk), streptomycin (10 μg per disk), tetracycline (30 μg per disk), chloramphenicol (30 μg per disk), erythromycin (15 μg per disk), co-trimoxazole (1.25 μg of trimethoprim and 23.75 μg of sulfamethoxazole per disk), rifampicin (25 μg per disk), and metronidazole (15 μg per disk). MRS agar plates containing 106–107 CFU mL−1 of the investigated Lactobacillus spp. strains were prepared after cultivation in MRS broth at 37 °C for 48 h. The disks were applied on the plates, subsequently incubated at 37 °C for 24 h. Inhibition zones around the disks were measured (mm) to evaluate the strain sensitivity (Charteris et al. 1998). The test was performed in triplicate.
In addition, a minimum inhibititory concentration (MIC) was determined for penicillin, ampicillin, vancomycin, gentamicin, tetracycline, erythromycin, clindamycin, and metronidazole by MICE Test strips (Oxoid). MRS agar plates containing 106–107 CFU mL−1 Lactobacillus spp. were prepared with previously cultured strains for 48 h at 37 °C in MRS broth. The antibiotic strips impregnated with gradient of antimicrobials were applied to the plates, subsequently incubated at 37 °C for 24 h. Inhibition zones around the strips were recorded and MIC determined according to the manufacture instructions. The test was performed in triplicate.
2.5.2 Genes for virulence, biogenic amines, and antibiotic resistance
The selected Lactobacillus spp. were tested for presence of virulence genes gelE (gelatinase), hyl (hyaluronidase), asa1 (aggregation substance), esp (enterococcal surface protein), cylA (cytolisin), efaA (endocarditis antigen), ace (adhesion of collagen), vanA and vanB (both related to vancomycin resistance), and genes for amino acid decarboxylases: hdc1 and hdc2 (both related to histidine decarboxylase), tdc (tyrosine decarboxylase), and odc (ornithine decarboxylase), using PCR protocols of Perin et al. (2014). The amplified products were separated by electrophoresis on 0.8 to 2.0% (w/v) agarose gels in 0.5× TAE buffer. Gels were stained in 0.5× TAE buffer containing 0.5 μg.mL−1 ethidium bromide (Sigma-Aldrich).
3 Results and discussion
3.1 Strain identification and characterization
More than 150 isolates were selected from 12 samples of Coalho cheeses produced from regions of Jaguaribe (Ceará State, Brazil). All 34 rod-shaped isolates previously identified as Lactobacillus spp. based on API50CHL fermentation profile were subject to RAPD-PCR differentiation analysis, which detected 17 genetically distinguished isolates. After selection of the isolates presenting unique genetic profiles by RAPD-PCR analysis, and combined with 16S rDNA and 16–23s intergenic spacer region sequencing analysis, 28 different Lb. rhamnosus strains, 5 Lb. plantarum strains, and a single Lactobacillus fermentum strain were identified (Fig. 1).
Differentiation of isolated Lb. rhamnosus, Lb. plantarum and Lb. fermentum based on RAPD-PCR analysis and 16S rDNA and 16–23s intergenic spacer region sequencing analysis. Twenty-eight Lb. rhamnosus strains, five Lb. plantarum, and one Lb. fermentum strains were identified. The strains selected for future study are indicated in bold
3.2 Strain selection and further characterization based on beneficial properties
3.2.1 Resistance to simulated gastric and intestinal conditions
Eight isolates—five identified as Lb. rhamnosus and three as Lb. plantarum strains—were selected for further investigation of potentially beneficial properties based on their resistance to GIT simulated conditions (Table 1). According to the FAO/WHO (2006), probiotics are defined as “life microorganisms which administrated in adequate amounts confer a health benefit on the host”; however, studies from the last two decades showing that even dead cells, cells particles, or metabolites can have health-promoting effects. Based on the definition of the FAO/WHO (2006), it is well recognized that the ability to survive through the GIT passage and to temporally persist in the host intestinal environment is the main functional characteristics for a probiotic strain. These features are considered critical to the health-promoting functions of probiotics (Kaushik et al. 2009).
The eight selected Lactobacillus spp. strains presented an SR based on the changes in the log order near or higher than 50% at the end of the in vitro assay. A remarkably high resistance to conditions simulating passage through the GIT was registered for Lb. rhamnosus EM1127 and Lb. rhamnosus EM1107, which showed an SR higher than 70%. Cell count reduction of 1.80 log CFU mL−1 in average was recorded for Lb. rhamnosus EM1127 after consecutive exposure to gastric and small intestine conditions, and a reduction of 2.46 log CFU mL−1 was registered for the Lb. rhamnosus EM 1107 population throughout the entire assay.
The gastric secretion is considered the primary defense mechanism against microorganisms, and resistance to the gastric passage is a rare property among the LAB (Cotter et al. 2005). Bile salts secreted into the small intestine also represent a challenge for bacterial survival in the GIT (Ouwehand et al. 2005). Meanwhile, our results show that the gastric phase was determinant of the final SRs observed for all studied strains, and the majority resisted well to the simulated enteric fluid containing 0.5% bile salts, with a registered recovery for the strains Lb. plantarum EM270 and Lb. plantarum EM472 after the enteric phase.
3.2.2 Bile salt deconjugation
All the eight Lb. plantarum and Lb. rhamnosus strains were able to grow on MRS agar plates containing 0.5% (w/v) sodium salts of TC, TDC, GC, and GDC, except Lb. rhamnosus EM253 and EM1025 and Lb. plantarum EM472 and EM480 on GDC. However, only Lb. rhamnosus EM1107 showed ability to deconjugate all the tested bile salts (Table 2). Deconjugation of bile salt is a common feature for Enterococcus spp. (dos Santos et al. 2014a), and this is not surprising, since enterococci are frequently reported as a part of GIT microbiota. However, Lactobacillus spp. are usually isolated from non-GIT environment, and such activity is not essential for their life cycle or survival. Meanwhile, in addition to the ability to survive in the presence of bile salts, the ability to deconjugate them is considered a desirable property in the selection of potentially probiotic bacteria since it can contribute to the reduction of serum cholesterol levels in the host (Begley et al. 2006). As the resulting bile acids are less soluble than the respective conjugated form, their intestinal absorption is lower, increasing their excretion in feces and the synthesis of new bile salts, which requires cholesterol. Besides, bile salt deconjugation activity can improve probiotic bacteria survival in the intestinal environment, increasing the possibility of exerting the beneficial effects (Begley et al. 2006). The ability of Lb. plantarum strains to perform bile salt deconjugation in vitro was reported by Kaushik et al. (2009), and Kumar et al. (2011) observed the antihypercholesterolaemic effects of two Lb. plantarum strains in rats. In addition, several clinical studies demonstrated cholesterol lowering effects of bile salt hydrolase (BSH)—active probiotic bacteria in vivo (Jones et al. 2013).
3.2.3 β-Galactosidase activity
An intense yellow color was observed on the test for two Lb. plantarum strains (Lb. plantarum EM270 and Lb. plantarum EM480), as well as for Lb. rhamnosus EM306, as a result of production of β-galactosidase enzymatic activity. All other strains except Lb. rhamnosus EM1025 also showed positive results, although producing a less intense yellow on the test. Dairy products containing lactobacilli and/or other microorganisms that produce β-galactosidase were used to alleviate lactose intolerance, a condition that is spread worldwide among adult population, through intraintestinal hydrolysis of lactose. β-Galactosidase activity is considered a positive feature in probiotic strains (Belicová et al. 2013) and starter cultures, giving them an advantage for growth and proliferation in milk environment. In addition, the ability to ferment lactose is an important technological property for Lactobacillus spp. to be employed in the dairy industry, as it has implications for the sensory attributes of dairy products. Presence of β-galactosidase activity in different LAB was previously reported (Todorov et al. 2010), and this is not surprising, since most of the LAB can grow on milk-based medium.
3.2.4 Hydrophobicity
Lb. plantarum and Lb. rhamnosus studied strains showed variable values in the hydrophobicity test, ranging from 5.4% in average for Lb. plantarum EM472 to 43.4% for Lb. rhamnosus EM1127. Among the studied strains, only Lb. rhamnosus EM1127 exhibited a moderate to high cell surface hydrophobicity (43.4%), determined as adhesion to N-hexadecane (Table 2). Adherence in the GIT is determinant for colonization and an extended residence time in the host, being considered one of the main selection criteria for potential probiotics (Kaushik et al. 2009). The hydrophobic nature of the cell surface was related to microorganism ability to adhere to intestinal epithelial cells, even though it is not a prerequisite for strong adherence, as the process of microbial adhesion to host tissue involves several mechanisms. The determination of microbial adhesion to N-hexadecane is considered a valid qualitative phenomenological approach to estimate the ability to adhere to epithelial cells (Kiely and Olson 2000). A hydrophobic cell surface promotes a nonspecific interaction between microbial cells and host, weak and often reversible, which might precede specific mechanisms involving cell surface proteins and lipoteichoic acids (Ross and Jonsson 2002).
Hydrophobicity varies among genetically closely related species and even among strains of the same species, as highlighted by Schar-Zammaretti and Ubbink (2003). Caustic et al. (2009) registered hydrophobicity values of 37.7% in average for Lb. plantarum Lp9, an indigenous strain isolated from buffalo milk, while we obtained values between 5.4 and 18.7% for the three studied Lb. plantarum strains (Table 2). Hydrophobicity values as high as 75–80% were reported by Todorov et al. (2008) for strains Lb. rhamnosus ST461BZ, Lb. rhamnosus ST462BZ, and Lb. plantarum ST664BZ. The same authors observed that some strains with a high cell surface hydrophobicity did not adhere at a high level to HT-29 cells, which is considered the gold standard for in vitro evaluation of microbial adhesion. On the other side, strain Lb. pentosus ST712BZ, with a lower hydrophobicity (38%), showed a higher adhesion to HT-29 cells (63%).
3.2.5 Autoaggregation and coaggregation with Listeria monocytogenes strains
The aggregation between bacterial cells is considered a major factor in adhesion and biofilm formation thereof to various surfaces, such as the mucosa of the GIT. Cellular aggregation facilitates transient colonization contributing to the persistence of beneficial microorganisms in GIT and their health effects. Besides, coaggregation of LAB, particularly Lactobacillus spp., may be considered a positive feature since they can exert antagonistic effects against pathogens, such as L. monocytogenes, using various mechanisms, involving the production of antimicrobial compounds such as organic acids, hydrogen peroxide, and bacteriocins, competing for substrate (Chen et al. 2007). The autoaggregation of Lactobacillus spp. strains that ranged from to 28.8% for Lb. rhamnosus EM253 to 83.7% for Lb. plantarum EM480 is presented in Table 2. The three studied Lb. plantarum strains showed higher rates of autoaggregation and coaggregation with L. monocytogenes compared with the Lb. rhamnosus strains, and the strain Lb. plantarum EM270 presented autoaggregation values higher than 80%, and coaggregation with L. monocytogenes up to 60%. Kleerebezem et al. (2003) detected genes that encode several adhesion proteins to various components of the environment in the genome of Lb. plantarum WCFS1. The higher rates of autoaggregation and coaggregation to L. monocytogenes observed in this study for Lb. plantarum EM270 and Lb. plantarum EM480 are probably due to the presence of similar adhesion proteins to those found in the WCFSI strain.
3.2.6 Assessment of antagonism and preliminary characterization of the inhibitory metabolites
From all tested Lactobacillus spp. strains, only Lb. rhamnosus EM253 presented bacteriocinogenic potential and inhibited 15 from 33 L. monocytogenes from different serological groups and 17 from 24 Enterococcus spp. However, no activity was detected against tested Lactobacillus spp., Leuconostoc spp., and Lactococcus spp. (data not shown). This is in agreement with the well-accepted definition for bacteriocins as antimicrobial compounds usually active against closely related microorganisms (Cotter et al. 2005). Complete inactivation of bacteriocin produced by Lb. rhamnosus EM253 was observed after treatment of bacteriocin-containing cell-free supernatants with α-chymotrypsin, proteinase K, pepsin, trypsin, protease XIV, and papain, confirming its proteinaceous nature. Treatment of bacteriocin with catalase, lipase, and α-amylase did not result in any changes of antimicrobial activity, indicating that the inhibition recorded was not attributed to hydrogen peroxide and that carbohydrate or lipid moieties were not required for antimicrobial activity. The bacteriocin remained stable after 3 h of incubation at pH values between 2.0 and 10.0. Similar results were recorded by Todorov and Dicks (2006). No decrease in the bacteriocin activity was recorded after treatment at 100 °C for 180 min and at 121 °C for 20 min. Resistance to temperatures around or higher than 100 °C was reported previously for most bacteriocins, including those produced by strains of Lactobacillus spp. and specifically Lb. rhamnosus (Todorov and Dicks 2006). Bacteriocin produced by Lb. rhamnosus EM253 was also not affected after treatment with N-laurylsarcosine, NaCl, SDS, Triton X-100, Tween 20, Tween 80, and urea. Similar results were recorded for other bacteriocins as pediocin ST18 (Todorov and Dicks 2005) and bozacin B14 produced Lc. lactis subsp. lactic (Ivanova et al. 2000). Bacteriocins produced by Lb. rhamnosus ST461BZ and ST462BZ were resistant to treatment with SDS, Tween 20, urea, and EDTA but sensitive to Triton X-100, Triton X-114, and Tween 80 (Todorov and Dicks 2006).
Addition of the bacteriocin produced by Lb. rhamnosus EM253 to early logarithmic phase cells of L. monocytogenes 211 (3 h old; ca. OD600nm = 0.17) resulted in growth inhibition after 1 h (from addition of the bacteriocin), followed by complete inhibition for the following monitored interval (Fig. 2). Only 200 AU mL−1 bacteriocin activity was recorded after treating the cells with NaCl at low pH, suggesting that the bacteriocin EM253 adheres to the surface of the producer cells in very low levels. In contrast, no bacteriocin adhesion to the cell surface of the producer cell was recorded for Lb. rhamnosus ST461BZ and Lb. rhamnosus ST462BZ (Todorov and Dicks 2006). Similar results were reported for bozacin B14 (Ivanova et al. 2000). However, in the case of plantaricin C19, maximal adsorption to cells was recorded between pH 5.0 and 7.0, but complete loss of adsorption was observed at pH 1.5 and 2.0 (Atrih et al. 1993).
Detectable levels of bacteriocin EM253 were recorded 7 h after inoculation (Fig. 3), indicating that expressed antimicrobial peptides are the primary metabolite. Similar results were reported for bacteriocins produced by Lb. rhamnosus ST461BZ and Lb. rhamnosus ST462BZ (Todorov and Dicks 2006). Production of bacteriocin EM253 occurred throughout logarithmic growth and was stabilized at 800 AU mL−1 during the next 12 h of slow growth (Fig. 3) and decrease to 400 AU mL−1 at 22 h from the beginning of the fermentation process. Extended growth does not necessarily lead to higher levels of bacteriocin activity. A decrease in activity levels after logarithmic growth was observed for other bacteriocins (Todorov and Dicks 2006). Very frequently, loss of activity was ascribed to proteolytic degradation, protein aggregation, adsorption to cell surfaces, and feedback regulation (De Vuyst et al. 1996; Todorov and Dicks 2006).
3.3 Technological characterization
3.3.1 Milk growth and viability in acid milk
All the studied Lactobacillus spp. strains were able to grow in milk, as expected considering their dairy origin. The strains Lb. plantarum EM472, Lb. plantarum EM480, and Lb. rhamnosus EM1127 were considered moderate acid producers, as they acidified milk to pH < 5.3 after 6 h of incubation. Rapid acidification is a desirable characteristic in the selection of LAB to be employed in starter cultures, which are responsible for accelerating and steering the fermentation process. Meanwhile, they can contribute to the development of desirable flavor and texture in fermented dairy products, as the intermediates of lactic acid production can originate flavor compounds (van Kranenburg et al. 2002). Probiotic bacteria are usually added to fermented food products as adjunct cultures, but they may have an additional role as a coculture with the starter.
The eight selected strains maintained good viability in acidified milk (pH 4.0 and 5.0) after 30 days of cold storage, showing very low decreases in population and even small increases (data not shown). The highest decrease, of 0.4 log CFU mL−1, was recorded for the population of Lb. plantarum EM270 and Lb. rhamnosus EM253 in milk at pH 4.0, and an increase of 0.3 log CFU mL−1 was observed for Lb. rhamnosus EM1107 in milk at the same pH. Decreases in cell counts lower than 0.8 log CFU mL−1 are considered low, according to Vinderola et al. (2008). Considering the generally low pH of fermented dairy foods, the ability to survive in acidified milk may be an interesting characteristic for probiotic candidates, related to the cold storage of these products. Georgieva et al. (2009) also determined the viability of Lb. plantarum strains in sterile skim milk during 2 months of cold storage and reported a populations ranging from 6.8 to 7.5 log CFU mL−1 until 28 days.
3.3.2 Bacterial interactions
Positive or antagonistic interactions between the Lb. plantarum and Lb. rhamnosus strains and commercial cultures usually employed in dairy processing were studied. It was observed that the cell-free supernatants of the tested commercial starter cultures presented no effect on the growth of the studied Lactobacillus spp. strains (data not shown). On the other hand, the cell-free supernatants of all Lactobacillus spp. strains presented a slight inhibitory effect on the growth of the starter cultures R-704 (Danisco), composed by Lc. lactis subsp. lactis and Lc. lactis subsp. cremoris, and St. thermophilus TA-040 (Danisco)—except Lb. rhamnosus EM1107 which showed no effect on R-704 growth and Lb. rhamnosus EM1025 which demonstrated a strong inhibitory effect on the same starter culture. Vinderola et al. (2008) also reported that strains of St. thermophilus and Lc. lactis were inhibited by the cell-free supernatant of Lactobacillus spp. isolated from feces of newborn infants, attributing the effect to the acidic character of the supernatant.
3.4 Safety evaluation
3.4.1 Susceptibility to antimicrobials
Lactobacilli are generally recognized as safe due to their long history of use in food fermentations. The safety of Lb. rhamnosus and Lb. plantarum, the species investigated in the present study, were recently confirmed as safe by the European Food Safety Authority in the updated 2012 Qualified Presumption of Safety (QPS) list (EFSA 2012). However, the selection of lactobacilli strains for food application as lactic cultures demands the evaluation of at least two important safety aspects, the resistance toward antimicrobials and the potential production of biogenic amines.
Antimicrobial resistance in genus Lactobacillus varies with the species and may be an inherent feature or a characteristic acquired through the exchange of genetic material, mutations, and the incorporation of new genes (Ammor et al. 2007). Resistance to glycopeptide antibiotics like vancomycin is common among Lactobacilli as highlighted by Franz et al. (2005) and described as intrinsic; a high-level of resistance (MIC values >256 μg.mL−1) was detected in our study for all the Lb. rhamnosus and Lb. plantarum strains except to Lb. plantarum EM270 (Table 3). All strains also showed high-level resistance to metronidazole, and the Lb. rhamnosus strains were resistant to streptomycin (except Lb. rhamnosus EM306). Danielsen and Wind (2003) also found resistance toward these two antibiotics among the majority of Lb. rhamnosus and Lb. plantarum strains they investigated.
On the other hand, as reported by Franz et al. (2005), less than 10% of the Lactobacilli used as commercial starter cultures showed resistance toward ampicillin, penicillin, erythromycin, and tetracycline, although most of them showed resistance toward aminoglycoside antibiotics like gentamicin and streptomycin. All the strains investigated here demonstrated susceptibility to penicillin G, ampicillin, erythromycin, clindamycin, and tetracycline, with an MIC below the breakpoint established by EFSA (2012), as well as to rifampicin and chloramphenicol—based on the disk diffusion method (Table 3). Meanwhile, Lb. plantarum EM480 and all Lb. rhamnosus strains except the strain EM306 were resistant toward gentamicin and streptomycin, with an MIC value above the EFSA breakpoint, as detected by the disk diffusion method. Resistance toward cotrimoxazole was also observed using the disk diffusion method for all studied Lb. rhamnosus except the strain EM1025, as well as for Lb. plantarum EM270 and EM480. The MIC breakpoint for this antibiotic for Lb. plantarum and Lb. rhamnosus was not established by EFSA (2012).
3.4.2 Genes for virulence, biogenic amines, and antibiotic resistance
Results concerning the presence of genes associated with virulence factors, production of biogenic amines, and vancomycin resistance are shown in Table 4. Virulence factors may be either colonization factors, such as those that promote the adhesion of bacteria to the host cells, or invasion factors that promote the invasion of epithelial cells, affecting the immune system (de Sousa 2003). Despite their general recognition of safety, the investigation of virulence factors in lactobacilli with potential application in food products is relevant due to the risk of genetic transfer to intestinal pathogens, as these genes are usually located in transferable plasmids (Eaton and Gasson 2001).
None of the investigated lactobacilli strains presented the targeting genes for aggregation substance (asa), cytolisin (cyt), gelatinase (gel), and endocarditis antigen (efa), which are associated with factors involved in colonization or tissue invasion (Hendrickx et al. 2009), nor the genes encoding resistance toward vancomycin (van A and van B), which in enterococci can be located in transferable plasmids (Courvalin 2006). Meanwhile, PCR targeting hyaluronidase (hyl) generated positive results for the three studied Lb. plantarum strains; however, adhesion of collagen (ace) was detected in Lb. rhamnosus EM253 and the three studied Lb. plantarum strains. ACE proteins facilitate the binding to collagen and may have a role during human infections, as well as hyaluronidase which facilitates the spread of bacteria and toxins through enzymatic degradation of host tissue (Girish and Kemparaju 2007). The implications of hyaluronidase activity among lactobacilli, however, are not clear as this has not been reported within the context of virulence and pathogenicity yet (Franz et al. 2005). All Lb. rhamnosus strains except Lb. rhamnosus EM1107 were shown to possess the hdc1 gene, whereas none of them had the odc and tdc genes. The odc gene was detected only in Lb. plantarum EM480. The formation of biogenic amines throughout hystidine, tyrosine, and ornithine decarboxylation are of concern in fermented food products and are among the intrinsic properties related to the safety of lactobacilli (Franz et al. 2005). Histamine, in particular, which is commonly found in cheese and other fermented dairy products, was reported responsible for allergic reactions.
The absence of all target genes for virulence factors and biogenic amines corroborates the safety of the Lb. rhamnosus EM1107. The expression of genes encoding virulence factors in strains Lb. rhamnosus EM306, EM1025, and EM1127 demands further investigation.
4 Conclusions
Taken together, the results of the in vitro tests performed to evaluate the beneficial properties and safety indicate Lb. rhamnosus EM1107 as a promising probiotic candidate to be applied in fermented dairy products, considering its elevated resistance to simulated GIT conditions and the ability to deconjugate bile salts, among other properties. Further studies are required to confirm the properties observed in vitro, and to further characterize Lb. rhamnosus EM1107. The results revealed the bacteriocinogenic potential of the strain Lb. rhamnosus EM253, which can have an application in dairy biopreservation, also deserve further investigation.
References
Ammor MS, Flóres AB, Mayo B (2007) Antibiotic resistance in not-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24:559–570
Atrih A, Rekhif N, Milliere JB, Lefebvre G (1993) Detection and characterization of a bacteriocin produced by Lactobacillus plantarum C19. Can J Microbiol 39:1173–1179
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738
Belicová A, Mikulášová M, Dušinský R (2013) Probiotic potential and safety properties of Lactobacillus plantarum from Slovak bryndza cheese. Biomed Res Int Article ID 760298, doi:10.1155/2013/760298
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot 61:1636–1643
Chen X, Xu J, Shuai J, Chen J, Zhang Z, Fang W (2007) The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int J Food Microbiol 115:307–312
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
Courvalin P (2006) Vancomycin resistance in gram-positive cocci. Clin Infect Dis 42:S25–S34
Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82:1–11
de Sousa CP (2003) Pathogenicity mechanisms of procaryotic cells: evolutionary view. Braz J Infect Dis 7:23–31
De Vuyst L, Callewaert R, Crabbe K (1996) Primary metabolite kinetics of bacteriocins biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocins production under unfavourable growth conditions. Microbiology 142:817–827
dos Santos KMO, Vieira ADS, Salles HO, Oliveira JS, Rocha CRC, Bruno LM, Borges MF, Franco BDGM, Todorov SD (2014a) Safety, beneficial and technological properties of Enterococcus faecium isolated from Brazilian cheeses. Braz J Microbiol in press
dos Santos KMO, Vieira ADS, Rocha CRC, Lopes ACS, Bruno LM, Carvalho JDG, Franco BDG, Todorov SD (2014) Brazilian artisanal cheeses as a source of beneficial Enterococcus faecium strains: characterization of the bacteriocinogenic potential. Ann Microbiol. doi:10.1007/s13213-013-0789-4
Eaton TJ, Gasson MJ (2001) Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol 67:1628–1635
EFSA – European Food Safety Authority (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740
FAO/WHO (2006) Probiotics in food - health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper 85, Rome
Franz CMAP, Hummel A, Holzapfel WH (2005) Problems related to the safety assessment of lactic acid bacteria starter cultures in probiotics. Mitt Lebensm Hyg 96:39–65
Georgieva R, Iliev I, Haertlé T, Chobert J-M, Ivanova I, Danova S (2009) Technological properties of candidate probiotic Lactobacillus plantarum strains. Int Dairy J 19:696–702
Girish KS, Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943
Hendrickx APA, Willems RJL, Bonten MJM, van Schaik W (2009) LPxTG surface proteins of enterococci. Trends Microbiol 17:423–430
Ivanova I, Kabadjova P, Pantev A, Danova S, Dousset X (2000) Detection, purification and partial characterization of a novel bacteriocin substance produced by Lactococcus lactis susp. lactase B14 isolated from boza—Bulgarian traditional cereal beverage. Biocatal Vestn Mosk Univ Kimia 41:47–53
Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S (2013) Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biolo Ther 13:631–642
Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S et al (2009) Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One 4(12):8099. doi:10.1371/journal.pone.0008099
Kiely LJ, Olson NF (2000) The physicochemical surface characteristics of Lactobacillus casei. Food Microbiol 17:277–291
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWWJ, Stiekema W, Lankhorst RMK, Bron PA, Hoffer SM, Groot MNN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100:1990–1995
Kumar R, Grover S, Batish VK (2011) Molecular identification and typing of putative probiotic indigenous Lactobacillus plantarum strain Lp91 of human origin by specific primed-PCR assays. Prob Antimicrob Prot 3:186–193
Lavilla-Lerma L, Perez-Pulido R, Martinez-Bueno M, Maqueda M, Valdivia E (2013) Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat’s milk cheeses. Int J Food Microbiol 163:136–145
Ouwehand C, Derrien M, de Vos W, Tiihonen K, Rautonen N (2005) Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol 16:212–217
Perin LM, Miranda RO, Todorov SD, Franco BDGM, Nero LA (2014) Virulence, antibiotic resistance and biogenic amines of bacteriocinogenic lactococci and enterococci isolated from goat milk. Int J Food Microbiol 185:121–126
Ross S, Jonsson H (2002) A high-molecular mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433–442
Salva S, Nunez M, Villena J, Ramos A, Font G, Alvarez S (2011) Development of a fermented goats’milk containing Lactobacillus rhamnosus: in vivo study of health benefits. J Sci Food Agric 91:2355–2362
Schar-Zammaretti P, Ubbink J (2003) The cell wall of lactic acid bacteria: surface constituents and macromolecular conformations. Biophys J 85:4076–4092
Silva RA, Lima MSF, Viana JBM, Bezerra VS, Pimentel MCB, Porto ALF, Cavalcanti MTH, Lima Filho JL (2012) Can artisanal “Coalho” cheese from Northeastern Brazil be used as a functional food? Food Chem 135:1533–1538
Song Y, Kato N, Liu C, Matsumiya Y, Kata H, Watanabe K (2000) Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett 187:167–173
Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Ng J, Munro K, Alatossava T (1999) Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl Environ Microbiol 65:4264–4267
Todorov S, Dicks LMT (2005) Pediocin ST18, an anti-listerial bacteriocin produced by Pediococcus pentosaceus ST18 isolated from boza, a traditional cereal beverage from Bulgaria. Process Biochem 40:365–370
Todorov SD, Dicks LMT (2006) Screening for bacteriocin producer lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria. Characterization of produced bacteriocins. Process Biochem 41:11–19
Todorov SD, Botes M, Guigas C, Schillinger U, Wiid I, Wachsman MB, Holzapfel WH, Dicks LMT (2008) Boza, a natural source of probiotic lactic acid bacteria. J Appl Microbiol 104:465–477
Todorov SD, Wachsman M, Tomé E, Dousset X, Destro MT, Dicks LMT, Franco BDGM, Vaz-Velho M, Drider D (2010) Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol 27:869–879
van Kranenburg R, Kleerebezem M, van Hylckama J, Ursing BM, Boekhorst J, Smit BA, Ayad EHE, Smit G, Siezen RJ (2002) Flavour formation from amino acids by lactic acid bacteria: predictions from genome sequence analysis. Int Dairy J 12:111–121
Vinderola G, Capellini B, Villarreal F, Suárez V, Quiberoni A, Reinheimer J (2008) Usefulness of a set of simple in vitro tests for the screening and identification of probiotic candidate strains for dairy use. LTW–Food. Sci Technol 41:1678–1688
Vizoso Pinto MG, Franz CMAP, Schillinger U, Holzapfel WH (2006) Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int J Food Microbiol 109:205–214
Wang Y, Cho JH, Chen YJ, Yoo JS, Huang Y, Kim HJ, Kim IH (2009) The effect of probiotic BioPlus 2B® on growth performance, dry matter and nitrogen digestibility and slurry noxious gas emission in growing pigs. Livest Sci 120:35–42
Yang R, Johnson M, Ray B (1992) Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol 58:3355–3359
Zago M, Fornasari ME, Carminati D, Burns P, Suàrez V, Vinderola G, Reinheimer J, Giraffe G (2011) Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol 28:1033–1040
Acknowledgments
To EMBRAPA for the financial support of this project (02.09.01.024.00). To Prof. Maria Teresa Destro and Dr. Eb Chiarini (University of São Paulo, São Paulo, Brazil) for providing L. monocytogenes strains used in this study. To Krysna Stephanny de Morais Ferreira, Jacqueline da Silva Oliveira, and Liana Maria Ferreira da Silva for providing technical assistance. Dr. Todorov received grants from CNPq (310203/2010-4) and FAPESP (2012/11571-6).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
dos Santos, K.M.O., Vieira, A.D.S., Buriti, F.C.A. et al. Artisanal Coalho cheeses as source of beneficial Lactobacillus plantarum and Lactobacillus rhamnosus strains. Dairy Sci. & Technol. 95, 209–230 (2015). https://doi.org/10.1007/s13594-014-0201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-014-0201-6