Abstract
Enhancing natural enemies for pest management in agriculture is an expanding approach offering new opportunities for pest control and the potential to reduce insecticide use. Numerous studies in a variety of cropping systems clearly have shown that adequate measures can benefit natural enemies. However, although carry-over effects from an increase in natural enemies and a subsequent decrease in pest populations leading to a reduction in crop damage are always assumed, they are rarely proven. We established an insecticide-free apple orchard optimized for the self-regulation of pests by supporting natural enemies with shelter, nectar, alternative prey/hosts, and pollen. For six growing seasons, we focused on the control of the major apple pest Dysaphis plantaginea. While fruit damage after the second fruit drop was not affected by aphidophagous insect guilds, it was negatively related to spider abundance in the previous autumn, when aphids immigrate back to the orchard to establish the next generation. In detail, we found that an increase in spider web area reduced the number of aphid fundatrices in spring and subsequently fruit damage. Our findings indicate the rarely proven carry-over effect of enhanced natural enemies on decreased crop damage and we show for the first time, how the rosy apple aphid can be managed without the use of insecticides.
Similar content being viewed by others
1 Introduction
Promoting natural enemies to reduce pest populations in agriculture is a promising approach gaining increasing recognition as an alternative to conventional pest control using insecticides (Letourneau and Bothwell 2008; Pfiffner and Wyss 2004). Viable alternatives to high insecticide use are in great demand because of major implications of pesticides for ecosystems and human health (Bourguet and Guillemaud 2016). The value of pest management in agricultural and horticultural production using natural enemies has been estimated to be worth more than 400 billion US$ per year globally, thus provides an immense ecosystem service free of charge (Van Lenteren 2006). There are several opportunities to unlock the potential of populations of natural enemies for crop protection, such as conserving non-crop habitats in the adjacent landscape and the establishment of flower strips and hedges alongside crop plants (Minarro et al. 2005; Pfiffner and Wyss 2004; Simon et al. 2010). These approaches increase nectar and pollen availability, enhance alternative prey to bridge gaps of low pest abundance, and offer shelter and overwintering sites to attract and enhance natural enemies.
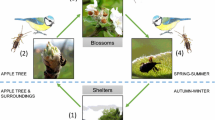
Many studies conducted in a variety of cropping systems show that promoting food resources and habitat quality can benefit natural enemies (Letourneau and Bothwell 2008; Pfiffner and Wyss 2004; Tschumi et al. 2016). However, Letourneau and Bothwell (2008) and Simon et al. (2010) point out that there are clearly fewer studies, which assess effects of natural enemy enhancement on populations of pest insects. For instance, Wyss et al. (1995) showed that flower strips in apple orchards increase the number of spider webs, resulting in decreased numbers of aphids, since spider webs catch aphids migrating to the apple orchards to establish the next generation (Fig. 1). However, studies linking promotion of natural enemies to crop damage or yield are even scarcer. Carry-over effects from an increase in natural enemies and a subsequent decrease in pest populations leading to a reduction in crop damage or to an increased yield are always assumed, but rarely proven (Letourneau and Bothwell 2008). In a few studies, those carry-over effects were discussed (Balzan et al. 2016; Tschumi et al. 2016). For example, flower strips sown nearby winter wheat decreased cereal leaf beetle abundance and consequently reduced pest-induced crop damage (Tschumi et al. 2016). However, not all studies could show this positive effect on crop damage. Sown flower strips in tomatoes increased the abundance and diversity of natural enemies, but did not significantly reduce crop damage (Balzan et al. 2016). There is evidence that pest control does not just depend on increased biodiversity or natural enemy abundance per se, but on promoting the “right biodiversity at the right time” (Letourneau and Bothwell 2008; Pfiffner and Wyss 2004). As a result, Letourneau and Bothwell (2008) emphasized the need for a better understanding of the effects of enhanced natural enemies on pest suppression. Further research evaluating not only the relationship between promoting natural enemies and pests, but also the effects on crop damage and yield is needed to constitute the ultimate aim of promoting natural enemies.
Orchards provide a suitable system to study crop protection by promoting natural enemies using tailored techniques of habitat management. They are perennial, which implies a certain stability and resilience, and they have a complex multi-strata structure (Fig. 1) offering diverse niches to enhance natural enemies (Nilsson et al. 2016; Pfiffner and Wyss 2004; Simon et al. 2010). The importance of ecosystem services provided by natural enemies has been pointed out for decades in orchards (Simon et al. 2010). The majority of efforts to promote natural enemies in apple orchards have been directed towards aphid antagonists (Dib et al. 2010; Nilsson et al. 2016). The rosy apple aphid Dysaphis plantaginea Passerini (Homoptera: Aphididae) is the most abundant and most damaging aphid in apples (Dib et al. 2010; Minarro et al. 2005). D. plantaginea provokes regular insecticide applications also in organic farming (Cross et al. 2007). In organic apple production, the use of synthetic pesticides and fertilizers is not allowed. Pest and disease control is therefore more challenging than in conventional production. However, despite the potential to control this important pest by promoting aphid antagonists, most organic apple orchards are not specifically designed to improve the self-regulation of aphids and still rely on biological insecticides. Therefore, we designed an innovative apple orchard optimized for self-regulation of pests by supporting natural enemies with shelter, nectar, alternative prey/hosts, and pollen including a variety of different intra-crop measures and adjacent to the orchard. Furthermore, no insecticides were used in order not to harm beneficial arthropods. In the present study, we focused on the carry-over effects of aphid antagonists on fruit damage by D. plantaginea.
2 Material and methods
2.1 Site description and experimental layout
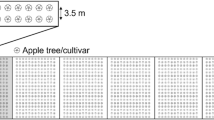
The experimental orchard (1 ha, Fig. 2) was planted in November 2006 in Frick, Switzerland (47° 30′ N, 8° 1′ E), in a medium to heavy brown soil with 33–49% clay, 30–49% silt, 0.6–4.2% humus, and a pH level of 7.4–8.0. The soil is poorly drained and prone to partly water-logging during winter and spring. Mean annual rainfall is about 1000 mm and mean temperature 8.9 °C.
Drawing of the experimental orchard. Small circles indicate individual trees of the cultivars Opal (O), Ecolette (E), Ariwa (A), and Topaz (T), planted with a distance of 1.5 m between single trees and 4 m between tree rows. The dark gray rectangle in the center represents a hedge with edible fruit (3 m width), light gray rectangles represent natural hedges (consisting of 19 native bush species), and cross-hatched rectangles represent extensively managed flower strips to connect the hedges with the tree rows (3 m width). In addition to those flower strips, low-growing, rosette-building flowering plants were planted underneath the trees and species-rich flower mixtures (ecotypes found in the Swiss Jura Mountains) in the alleyways. Four sampling blocks were placed within the orchard
One-year-old trees and hedges were planted in 2006 and flower strips in 2007. With a distance of 8.4 m to the edge of the orchard and 20 m to each other, four assessment blocks of 20 × 20 m were placed in the orchard. Each block included two apple cultivars planted in five rows with row distances of 4 m and a distance of 1.5 m between single trees.
2.2 Characteristics of the experimental orchard
In orchards on organic farms, mown grass strips in the alleyways are considered necessary for permanent accessibility by tractors; high yielding cultivars are selected based on market demands (also highly susceptible to apple scab); low growing rootstocks (M9) are used to simplify workflow; cultural practices (pruning, thinning, fertilization, irrigation, weed management) focus on yield maximization; biocontrol products or biological and mineral insecticides and fungicides are applied for pest and disease control.
The innovative, experimental orchard presented in this paper differed in many ways from a standard organic orchard, because it was optimized for self-regulation using the measures described in Table 1. The aim was to achieve a maximum impact of natural enemies on insect pests, to avoid diseases by using scab-robust cultivars and to achieve self-sufficiency in tree nutrition.
2.3 Arthropod sampling
In the present study, we focus on aphid antagonists and their effects on the rosy apple aphid D. plantaginea and the associated fruit damage in the first 6 years of full yield from 2009 to 2014.
Spider abundance in the previous autumn was assessed shortly before harvest (2008–2013). In both apple cultivars in all four blocks, 50 randomly selected fruit and 50 long shoots were visually assessed for the number of spiders. Shortly before flowering (BBCH 56–60) in April 2009–2014, 50 randomly selected flower clusters of both apple cultivars in all four blocks were visually assessed for the number of fundatrices of D. plantaginea and for aphid antagonists (Coccinellidae, Syrphidae, Anthocoridae, Miridae, Chrysopidae, Cecidomyiidae, and Forficulidae). We assessed eggs, larvae, pupae, and adults of beneficial insects, except for Syrphidae, Cecidomyiidae, and Chrysopidae, where we assessed no adults, because they do not have direct impact on the target pest. After the second fruit drop in late June 2009–2014, 50 randomly selected fruit clusters of both apple cultivars in all four blocks were visually assessed for characteristic D. plantaginea fruit damage, such as malformation and reduction in fruit size. We did not consider flower damage resulting in flower abortion, since flower number needs to be regularly reduced (flower thinning) in order to optimize fruit load per tree. Additionally, excessive honeydew production was never an issue in our orchard. Furthermore, 50 long shoots of both apple cultivars in all four blocks were examined for presence of aphid antagonists. Because spiders were unexpectedly the most abundant beneficial arthropods found in the first year of assessment, we evaluated spiders in more detail in the previous autumn in all following years (2009–2013): Spider web diameter (cm) of Araneidae and Tetragnathidae to reflect spider web area in the previous autumn was measured on 50 randomly selected branches for both apple cultivars in all four blocks.
2.4 Data analysis
We used R 3.3.1 and the R-package lme4 to perform mixed effect models. Visual inspections of residual plots were applied to test for any obvious deviations from homoscedasticity or normality and temporal autocorrelation. The best fitting random effect structure and the inclusion of the random effect block and/or year were based on the Akaike information criterion (AIC) and likelihood ratio tests. Non-significant interactions were removed in step-wise model simplification processes and decisions were based on likelihood ratio tests.
To assess effects of different beneficials on the number of damaged fruits after the second fruit drop (2009–2014), a generalized linear model with Poisson-distributed errors was used. The model included the random factor block and an observation level (a random factor with the levels 1 to n) (Korner-Nievergelt et al. 2015) to handle overdispersion and the fixed variables apple cultivar (Ariwa and Topaz), the number of aphid fundatrices in spring, spider abundance from the previous autumn, and the number of beneficial insects in spring and summer.
To analyze effects of spider web area on the number of aphid fundatrices in spring (2010–2014), a generalized linear mixed effect model with Poisson-distributed errors was used. The model included the random factor year and the fixed variables apple cultivar (Ariwa and Topaz) and the diameters of Araneidae and Tetragnathidae webs from the previous autumn. To make the estimate of the effect sizes between different spider families comparable, web diameters were scaled by dividing diameters by the standard deviation.
To evaluate effects of spider web area on fruit damage after the second fruit drop (2010–2014), a generalized linear model with Poisson-distributed errors was used. The model included the random factor block and the fixed variables apple cultivar (Ariwa and Topaz), the number of aphid fundatrices in spring, and the diameters of Araneidae and Tetragnathidae webs from the previous autumn. To make the estimate of the effect sizes between different spider families and aphids comparable, web diameters and aphid abundance were scaled by dividing values by the standard deviation.
3 Results and discussion
3.1 Reduction of aphid damage through web-building spiders
The fruit damage by aphids decreased with a higher abundance of web-building spiders in the previous autumn (z = − 2.8, P = 0.005). An increase of the spider population by 10.5% decreased fruit damage by 11.0%. Spider webs catch flying D. plantaginea migrating back to the orchard from the obligate summer host Plantago spp. (Lampel 1968), and therefore, prevent aphids from establishing the next aphid generation, which damages fruits in the following year. Wyss et al. (1995) showed that flower strips in apple orchards increase the number of spider webs from Araniella spp. (Araneidae). The higher numbers of spider webs resulted in decreased numbers of overwintering aphid eggs, and thus, decreased numbers of aphids in spring. In our orchard, Araneidae were clearly the most abundant spiders (80%) and their spider web area affected aphids and the subsequent fruit damage. For instance, there was a negative interaction between Araneidae and Tetragnathidae web area on aphid fundatrices in spring (z = − 2.7, P = 0.007). A simultaneous increase of 10.2 and 41.3% in web area of Araneidae and Tetragnathidae, respectively, decreased the number of aphid fundatrices in spring by 10.0%. Furthermore, the suggested multi-level mechanism of spider webs reducing immigrating aphids in fall, resulting in a reduced pest pressure in spring and subsequently reduced fruit damage in summer, was reflected by the significant interactions between aphids and Araneidae web area (z = − 2.3, P = 0.021) and aphids and Tetragnathidae web area (z = − 3.4, P < 0.001) on fruit damage. An increase of 10.1 or 26.5% in web area of Araneidae and Tetragnathidae, respectively, decreased aphid fruit damage by 10.0%. The interactions between spider web area and aphid fundatrices on fruit damage clearly indicate the carry-over effect of enhanced natural enemies on decreased crop damage via the control of pest abundance. Although this multi-level effect has rarely been shown, it is indispensable to demonstrate the benefit of promoting natural enemies for pest control and a subsequent reduction in crop or yield damage (Letourneau and Bothwell 2008).
In contrast, effects of spiders on wingless, non-migrating D. plantaginea in spring are inconsistent (Dib et al. 2010). Generally, spider webs catch very efficiently small and rather slow flying insects with a relatively large wing surface (Nentwig 1987) and are therefore often effective in pest control, even if certain spider species particularly focus on other prey types. Furthermore, web-building spiders capture and kill 50 times more pest insects than they eventually consume (Kajak 1978), since they can secure and store prey before ingestion (Riechert and Lockley 1984) and even abandoned spider webs still catch prey. This emphasizes that web-building spiders are highly effective aphid antagonists by catching migrating, winged morphs in autumn, resulting in less aphid fundatrices in the following spring. The promotion of web-building spiders is therefore a promising approach for crop protection in apple orchards. Previous studies showed that ground cover vegetation and flower strips in apple orchards increase spider abundance (Marliac et al. 2016; Wyss et al. 1995). Spiders benefit from higher habitat complexity and augmented alternative prey availability (Nilsson et al. 2016; Wyss et al. 1995). Furthermore, adjacent, natural habitats enable the colonization of orchards with spiders (Sackett et al. 2009). Therefore, we provided not only flowering plants within tree rows and in alleyways, but planted also hedges around the orchard and connected them to the trees via extensively managed flower strips. However, other studies have reported inconsistent results of flower strips on web-building spiders in conventionally managed orchards (Marko and Keresztes 2014). In another apple orchard at the same location, with the same apple cultivars, but with a standard management practice (including organic and mineral insecticides) and spatial design for organic apple orchards, spider abundance in autumn during the same assessment period was 40.2% smaller than in our study orchard. This reference shows the potential benefit of our implemented design to enhance beneficial arthropods, since we found that an increase of the spider population (mainly consisting of Araneidae) by 10.5% decreases fruit damage by 11.0%.
3.2 No reduction of aphid damage through beneficial insects
Several studies describe the guilds of natural enemies associated with insect pests in apple orchards, reporting of a minimum of 50 species of arthropods from various families (Pfiffner and Wyss 2004). Arthropod biodiversity (149 species) was relatively rich in our orchard (Table 2), since measures to promote natural enemies usually benefit not only one specific group, but numerous species. Thus, effects of specific aphidophagous predators could be additive or even synergistic (Snyder et al. 2005). Previous studies showed that Episyrphus balteatus De Geer (Syrphidae) could have an additive control effect on D. plantaginea together with Adalia bipunctata L. (Wyss et al. 1999) or Forficula auricularia L. (Dib et al. 2011). In contrast, negative effects of coexisting F. auricularia and E. balteatus through intra-guild predation could also reduce aphid control (Hindayana et al. 2001). However, we neither found significant effects of beneficials in spring (z = − 0.7, P = 0.474) nor summer (z = 1.8, P = 0.066) on fruit damage. In our orchard, E. balteatus was numerically the dominant aphid antagonist in spring. Episyrphus balteatus is often identified as one of the most promising aphid predators, because it occurs in early spring and feeds on fundatrices before they can build up large colonies (Dib et al. 2010; Minarro et al. 2005; Wyss et al. 1999). While several studies showed that E. balteatus is able to control aphid populations (Dib et al. 2010; Wyss et al. 1999), others found no effect (Minarro et al. 2005), indicating that the reduction of crop damage through E. balteatus is not reliable and might depend on spring climate conditions. More nectar-sensitive aphid predators were targeted with the diverse flower mixtures implemented in the orchard (Table 1). Although the nectar-sensitive Chrysopidae, for example, were numerically one of the most abundant beneficial insects in summer (Table 2), we found no significant effect of beneficials in summer on fruit damage. However, parasitation of aphids was negligible and nectar-sensitive parasitoids were not assessed in the present study.
Surprisingly, only spiders (generalist predator) had an impact on fruit damage, whereas the specialist aphid predators in spring, mainly represented by Syrphidae, had no effect. However, molecular techniques revealed that early season predation by generalist predators such as Coccinellidae, spiders, and Carabidae occur regularly and is probably most effective to prevent outbreaks of r-strategist pests before yield loss is induced (Athey et al. 2016).
3.3 Economic threshold for aphid control
At the first visual control in spring 2009–2014, 9.3 ± 1.8 fundatrices per 50 flower clusters were counted on the cultivar Ariwa and 3.6 ± 0.8 fundatrices per 50 flower clusters were observed on Topaz. Both numbers are clearly above the economic threshold of only 1–2 fundatrices per 100 flower clusters (Hemptinne et al. 2002). This low threshold is the main driver for regular neem applications even in organic orchards, because it suggests that D. plantaginea is extremely harmful. Generally, apple orchards are consistently sprayed with insecticides for aphid control, although aphids rarely remain abundant for several successive growing seasons. A strong aphid antagonist community could therefore manage aphid abundance on a low level and subsequently reduce insecticide applications, which also harm beneficial arthropods. However, economic thresholds for D. plantaginea are not experimentally determined (Whalon and Croft 1984) and do not consider the presence of aphid antagonists. These low economic thresholds for D. plantaginea are considered as a main factor hampering natural aphid control (Hemptinne et al. 2002).
Despite the absence of insecticides, we have not observed an unacceptably high fruit damage by D. plantaginea. Both cultivars showed a similar level of aphid damage (Ariwa 5.8 ± 0.6%; Topaz 6.0 ± 2.5%; z = 0.5, P = 0.6). However, since the number of aphid fundatrices in spring differed between cultivars, thresholds to control D. plantaginea should be cultivar-specific.
3.4 Limitations of the study orchard optimized for self-regulation of pests
Pesticides clearly harm and decrease spider populations (Marliac et al. 2016). Pesticide applications during summer, when spider populations start to develop, and in autumn, when spider webs catch migrating aphids, should therefore be avoided. In our orchard, measures for self-regulation of insect pests therefore also comprised the avoidance of pesticides, because even fungicides used in organic agriculture can harm natural enemies. However, the avoidance of fungicides resulted in a high incidence of sooty blotch and flyspeck (Schizothyrium pomi (Mont. & Fr.) Arx and Phyllachora pomigena (Schwein.) Sacc.), which led to a lower market quality of harvested fruits. Obviously, the lower tree density to prevent diseases (Table 1) did not pay off, and therefore, tree rows could be planted closer to each other to increase yield and lower production costs per hectare. In order to overcome the limiting factor sooty blotch, biocontrol strategies or tolerant varieties are needed to control this disease without interfering the self-regulation of aphids and other pests. Furthermore, since our focus was on aphid control, effects of the orchard design on other apple pests need to be investigated too. The self-regulation of aphids is therefore only one piece in a larger puzzle of sustainable apple production.
The study orchard was located in a structurally complex and small-scaled landscape, which may have positively affected beneficial arthropod populations due to immigration from the surrounding landscape (den Belder et al. 2002; Sackett et al. 2009; Thies and Tscharntke 1999). In orchards located in monotonous landscapes, elements implemented in the present study orchard may not be as effective, because it could last several seasons to build up powerful beneficial arthropod populations (Bostanian et al. 2004). However, pest-beneficial systems do not necessarily depend on the surrounding landscape complexity (Tschumi et al. 2015). Nevertheless, the present study shows for the first time that fruit damage by D. plantaginea can be managed without insecticides, but by promoting natural aphid antagonists in an apple orchard optimized for self-regulation of pests. Using natural elements within and adjacent to the orchard to promote aphid antagonists, namely spiders in autumn, could therefore be a suitable tool to keep aphid populations under control.
4 Conclusions
Spider abundance in the previous autumn was negatively related to fruit damage. Actually, larger spider web areas catch more immigrating aphids in autumn, resulting in lower numbers of aphid fundatrices in the following spring and subsequently lower fruit damage. The present study therefore presents the rarely shown carry-over effect of enhanced natural enemies on decreased crop damage via the control of pest abundance. However, adapted biocontrol strategies and/or tolerant varieties are needed to control diseases without harming beneficial arthropods and interfering the self-regulation of aphids and other pests. Nevertheless, our data assessed for 6 years indicate that the promotion of web-building spiders by natural elements within and adjacent to the orchard is a promising approach for crop protection against D. plantaginea in apple orchards.
References
Athey KJ, Dreyer J, Kowles KA, Penn HJ, Sitvarin MI, Harwood JD (2016) Spring forward: molecular detection of early season predation in agroecosystems. Food Webs 9:25–31. https://doi.org/10.1016/j.fooweb.2016.06.001
Balzan MV, Bocci G, Moonen AC (2016) Utilisation of plant functional diversity in wildflower strips for the delivery of multiple agroecosystem services. Entomol Exp Appl 158(3):304–319. https://doi.org/10.1111/eea.12403
Bostanian NJ, Goulet H, O'Hara J, Masner L, Racette G (2004) Towards insecticide free apple orchards: flowering plants to attract beneficial arthropods. Biocontrol Sci Tech 14(1):25–37. https://doi.org/10.1080/09583150310001606570
Bourguet D, Guillemaud T (2016) The hidden and external costs of pesticide use. Sustain Agric Rev 19:35–120. https://doi.org/10.1007/978-3-319-26777-7_2
Cross JV, Cubison S, Harris A, Harrington R (2007) Autumn control of rosy apple aphid, Dysaphis plantaginea (Passerini), with aphicides. Crop Prot 26(8):1140–1149. https://doi.org/10.1016/j.cropro.2006.10.007
den Belder E, Elderson J, van den Brink WJ, Schelling G (2002) Effect of woodlots on thrips density in leek fields: a landscape analysis. Agric Ecosyst Environ 91(1–3):139–145. https://doi.org/10.1016/s0167-8809(01)00264-x
Dib H, Simon S, Sauphanor B, Capowiez Y (2010) The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south-eastern France. Biol Control 55(2):97–109. https://doi.org/10.1016/j.biocontrol.2010.07.005
Dib H, Jamont M, Sauphanor B, Capowiez Y (2011) Predation potency and intraguild interactions between generalist (Forficula auricularia) and specialist (Episyrphus balteatus) predators of the rosy apple aphid (Dysaphis plantaginea). Biol Control 59(2):90–97. https://doi.org/10.1016/j.biocontrol.2011.07.012
Hemptinne J-L, Dixon AFG, Wyss E (2003) Biological control of the rosy apple aphid, Dysaphis plantaginea (Passerini) (Homoptera: Aphididae): learning from the ecology of the ladybird beetles. In: Soares AO, Ventura MA, Garcia V, Hemptinne J-L (eds) Proceedings of the 8th International Symposium on Ecology of Aphidophaga: Biology, Ecology and Behaviour of Aphidophagous Insects. Arquipélago, Life and Marine Sciences, Supplement: 33-41
Hindayana D, Meyhofer R, Scholz D, Poehling HM (2001) Intraguild predation among the hoverfly Episyrphus balteatus de Geer (Diptera : Syrphidae) and other aphidophagous predators. Biol Control 20(3):236–246. https://doi.org/10.1006/bcon.2000.0895
Kajak A (1978) Analysis of consumption by spiders under laboratory and field conditions. Ekologia Polska 26:409–427
Korner-Nievergelt F, Roth T, von Felten S, Guélat J, Almasi B, Korner-Nievergelt P (2015) Bayesian data analsis in ecology using linear models with R, BUGS, and Stan. Academic Press Elsevier, p 125
Lampel G (1968) Biologie des Blattlaus-Generationswechsels. VEB Gustav Fischer, Verlag, Jena
Letourneau DK, Bothwell SG (2008) Comparison of organic and conventional farms: challenging ecologists to make biodiversity functional. Front Ecol Environ 6(8):430–438. https://doi.org/10.1890/070081
Marko V, Keresztes B (2014) Flowers for better pest control? Ground cover plants enhance apple orchard spiders (Araneae), but not necessarily their impact on pests. Biocontrol Sci Tech 24(5):574–596. https://doi.org/10.1080/09583157.2014.881981
Marliac G, Mazzia C, Pasquet A, Cornic JF, Hedde M, Capowiez Y (2016) Management diversity within organic production influences epigeal spider communities in apple orchards. Agric Ecosyst Environ 216:73–81. https://doi.org/10.1016/j.agee.2015.09.026
Minarro M, Hemptinne JL, Dapena E (2005) Colonization of apple orchards by predators of Dysaphis plantaginea: sequential arrival, response to prey abundance and consequences for biological control. BioControl 50(3):403–414. https://doi.org/10.1007/s10526-004-5527-1
Nentwig W (1987) The ecophysiology of spiders. Springer, Berlin
Nilsson U, Porcel M, Świergiel W, Wivstad M (2016) Habitat manipulation – as a pest management tool in vegetable and fruit cropping systems, with the focus on insects and mites. SLU, EPOK – Centre for Organic Food & Farming. 07:12, http://orgprints.org/30032/
Pfiffner L, Wyss E (2004) Use of sown wildflower strips to enhance natural enemies of agricultural pests. In: Gurr GM, Wratten SD, Altieri MA (Hrsg.) Ecological engineering for pest management. CABI-Publishing, Collingwood, Australia, Kapitel 11, S. 167-188
Riechert SE, Lockley T (1984) Spiders as biological control agents. Annu Rev Entomol 29(1):299–320. https://doi.org/10.1146/annurev.en.29.010184.001503
Sackett TE, Buddle CM, Vincentb C (2009) Dynamics of spider colonization of apple orchards from adjacent deciduous forest. Agric Ecosyst Environ 129(1–3):144–148. https://doi.org/10.1016/j.agee.2008.08.005
Schmid A, Weibel FP (2000) Das Sandwich-System –ein Verfahren zur herbizidfreien Baumstreifenbe- wirtschaftung? [The Sandwich System, a procedure for herbicide free in-row weed control?]. Obstbau 25:214–217
Simon S, Bouvier JC, Debras JF, Sauphanor B (2010) Biodiversity and pest management in orchard systems. Rev Agronomy Sustain Dev 30(1):139–152. https://doi.org/10.1051/agro/2009013
Snyder WE, Chang GC, Prasad RP (2005) Conservation biological control: biodiversity influences the effectiveness of predators. In: Barbosa P, Castellanos I (eds) Ecology of predator-prey interactions. Oxford University Press, New York
Thies C, Tscharntke T (1999) Landscape structure and biological control in agroecosystems. Science 285(5429):893–895. https://doi.org/10.1126/science.285.5429.893
Tschumi M, Albrecht M, Entling MH, Jacot K (2015) High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc Royal Soc B-Biol Sci 282(1814):189–196. https://doi.org/10.1098/rspb.2015.1369
Tschumi M, Albrecht M, Baertschi C, Collatz J, Entling MH, Jacot K (2016) Perennial, species-rich wildflower strips enhance pest control and crop yield. Agric Ecosyst Environ 220:97–103. https://doi.org/10.1016/j.agee.2016.01.001
Van Lenteren J (2006) Ecosystem services to biological control of pests: why are they ignored? Proc Netherlands Entomol Soc Meet 17:103–111
Whalon ME, Croft BA (1984) Apple IPM implementation in North America. Annu Rev Entomol 29(1):435–470. https://doi.org/10.1146/annurev.en.29.010184.002251
Wyss E, Niggli U, Nentwig W (1995) The impact of spiders on aphid populations in a strip-managed apple orchard. J Appl Entomol 119(1-5):473–478. https://doi.org/10.1111/j.1439-0418.1995.tb01320.x
Wyss E, Villiger M, Muller-Scharer H (1999) The potential of three native insect predators to control the rosy apple aphid, Dysaphis plantaginea. BioControl 44(2):171–182. https://doi.org/10.1023/a:1009934214927
Acknowledgments
We thank Franco Weibel, Lucius Tamm, Eric Wyss, Ignazio Giordano, Andreas Hammelehle, Francisco Suter, Hansjakob Schärer, Andi Häseli, Simon Schweizer, Susanne Tesch, Jasmin Arab, Silvia Matray, Mathias Ludwig, Pius Allemann, Afred Schädeli, Bronya Dehlinger, Christian Vogt, Chloë Raderschall and Heinz Leutwyler from FiBL, and Jörg Samietz, Esther Bravin, Heinrich Höhn, and Andres Beck from Agroscope for their assistance in the project.
Funding
We thank the Bundesamt für Landwirtschaft, Pan-Civis Stiftung, Hans-Eggenberger-Stiftung, Paul Schiller-Stiftung, and Stiftung Dreiklang für ökologische Forschung und Bildung for the funding of the project.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cahenzli, F., Pfiffner, L. & Daniel, C. Reduced crop damage by self-regulation of aphids in an ecologically enriched, insecticide-free apple orchard. Agron. Sustain. Dev. 37, 65 (2017). https://doi.org/10.1007/s13593-017-0476-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-017-0476-0