Abstract
Xylocopa nigrocincta Smith, 1854, and Xylocopa suspecta Moure & Camargo, 1988, are two nominal species within the subgenus Neoxylocopa and have a sympatric geographic distribution in South America. While X. nigrocincta is recognized by the presence of reddish metasomatic bands, X. suspecta is entirely black. Although morphologically distinct in terms of metasomal band colour, other morphological characters suggest that both species could be the same evolutionary entity and therefore synonymous. The aim of this research was to review both nigrocincta and suspecta morphotypes using an integrative approach (morphological and molecular) to evaluate if they are truly two different evolutionary lineages. Females of both species were obtained from field collections and museums, representing a large part of their morphotype distribution. Additional diagnostic characters of the external morphology were investigated, such as metasomal band colour, metasomal punctuation, wing colours, and apical regions of the basitibial plate. Mitochondrial gene sequences (COI and CytB) were used for phylogenetic reconstructions. Our results showed that both nigrocincta and suspecta morphotypes are undistinguished based on morphology, although the metasomal band colour, together with the geographic distribution, revealed the presence of three distinct morphogroups, including an intermediate one with a variable number of reddish bands. Nonetheless, the three morphogroups are not supported by molecular data and therefore represent intra-specific variations. In conclusion, our results do not support the hypothesis that the two nominal species are distinct evolutionary lineages, and we propose a synonym between X. nigrocincta and X. suspecta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The genus Xylocopa Latreille, 1802 (Apidae, Xylocopini), is a common group of tropical large bees usually called mamangavas in Brazil or carpenter bees (Hurd and Moure 1963; Michener 2007). They build their nests digging dead wood or other plant materials. There are approximately 470 recognized species distributed in 31 subgenera, inhabiting mainly the tropical and subtropical climates of the world (Leys et al. 2002; Michener 2007). Carpenter bees are key species for pollination of cultivated plants from various groups, such as passion fruit, pumpkin, tomato, Brazilian nuts, and eggplants (Keasar 2010; Yamamoto et al. 2014; Giannini et al. 2015).
Among the 12 to 17 subgenera described in the Neotropical region, Neoxylocopa Michener, 1954, is the most diverse, with 50 recognized species (Michener 2007; Moure 2022). Within the nominal species of this subgenus, Xylocopa nigrocincta Smith, 1854, is easily distinguished by the presence of reddish metasomal bands, with variation in the number of bands (Schlindwein et al. 2003; Marchi and Alves-dos-Santos 2013; Lucia et al. 2014). In the original description of X. nigrocincta, based on a female specimen from South America without a specific location record, the author mentions the ferruginous metasoma and the tergal apical margins with black bands, but with a black apical segment, and the wings’ dark-fusco ferruginous with a splendid violet iridescence (Smith 1854). Hurd and Moure (1963) established the synonym with Xylocopa schultessi Dusmet & Alonso, 1924, described based on a specimen with similar morphological characteristics collected in the state of Rio Grande do Sul, Brazil. More recently, Lucia et al. (2014) used a similar diagnosis for specimens from Argentina, while Marchi and Alves-dos-Santos (2013) mentioned three or four reddish bands for individuals from São Paulo state. Schlindwein et al. (2003) only mentioned the presence of reddish bands without specifying the number.
In contrast to X. nigrocincta, Xylocopa suspecta Moure & Camargo, 1988, is entirely black, similar with the following sympatric species: Xylocopa ordinaria Smith, 1874, Xylocopa carbonaria Smith, 1854, and Xylocopa submordax Cockerel, 1935 (synonymy of Xylocopa transitoria Pérez, 1901). Characteristics such as body size, sparse punctuation on the disc of T2 with short pubescence, and wings with a greenish iridescence were highlighted for its recognition. However, there has been no direct comparison between X. suspecta and X. nigrocincta, possibly due to the clear absence of reddish metasomal bands in the former species. More recently, Lucia and Gonzalez (2017) claimed that X. nigrocincta and X. suspecta could be conspecific, as they considered male and female specimens tagged as X. suspecta and collected in Bolivia (El Beni) to be indistinguishable from X. nigrocincta, emphasizing the need for an investigation based on a larger number of individuals and a wider geographical representation.
A preliminary analysis of individuals previously identified as X. nigrocincta or X. suspecta allowed us to recognize the presence of wider variation in the number of reddish bands and other diagnostic characters. Along with their broad sympatric distribution, from Argentina to Central and Eastern Brazil (Moure 2022), these results prompted us to investigate whether both species, X. nigrocincta and X. suspecta, have different evolutionary histories or, alternatively, if they are synonyms. By combining morphological and molecular approaches, we reviewed the morphological characters of both species, and this analysis was complemented by molecular data from partial sequencing of Cytochrome C Oxidase Subunit I (COI) and Cytochrome B (CytB) genes. Our results revealed the presence of three different morphogroups based on band colour and geographic distribution, but these morphogroups were not supported by the molecular data, indicating that (i) these morphogroups represent intra-specific variation within this group, (ii) morphology is not reliable for species identification and delimitation, and (iii) X. nigrocincta and X. suspecta are the same species, leading us to propose them as synonyms.
2 Materials and methods
2.1 Biological samples
A total of 226 specimens (all females) were obtained from field collections in the states of Pará, Paraná, Minas Gerais and São Paulo, as well as from entomological collections (Supp 3). Males were excluded in the analysis due to the low representation in collection. For male diagnosis, please see Moure and Camargo (1988) and Lucia et al. (2014). The collected specimens were sacrificed with potassium cyanide and stored in labelled tubes containing 70% ethanol at −4 °C. The tubes were identified with information such as location, coordinate, date, collector, and collection method.
2.2 Morphology
All female specimens were screened and analyzed following identification keys for bees of the subgenus Neoxylocopa by Schlindwein et al. (2003), Marchi and Alves-dos-Santos (2013), Lucia et al. (2014) and Mawdsley (2018). The analysis was conducted using a stereoscopic Zeiss Discovery V8 microscope at the “Enedina Marques de Souza” Multi-User Laboratory (Itaipu/PTI/Unila). A comprehensive search was conducted on all specimens to record variation in external morphological characters, focusing on those previously mentioned in taxonomic works and the original descriptions by Smith (1854) and Moure and Camargo (1988). Images were captured using a Zeiss V12 microscope with Axion Vision SE 64.4 software. Whole-body specimen photos were taken using a Canon 6Di camera. The photos were edited using Adobe Photoshop CC 2017, and the image board was created using Adobe Illustrator CC 2017. Nomenclature for bee anatomy followed Michener (2007), Silveira et al. (2002), and Hurd and Moure (1963). Measurements were taken using a digital caliper, including TBL (total body length), MetL (metasomal length), MetW (metasomal width), MesL (mesosomal length), MesW (mesosomal width), HL (head length) and HW (head width). Additional structures were abbreviated as T (metasomal tergum) and BP (basitibial plate). The analyses considered two different morphotypes described in the literature based on band colour: the suspecta morphotype, entirely black, and the nigrocincta morphotype, with one or more reddish bands.
2.3 Map production
Geographic coordinates were obtained directly from specimen labels. When only the name of the municipality and locality were available, coordinates were obtained through consultation and plotted on the map using Google Earth Pro Version 9.3.3.1.5. The coordinates and location data were saved in KMZ format and converted into a Shapefile using the website http://www.zonums.com/online/kml2shp.php. The Shapefile was then exported to ArcMap 10.2.2 software for map construction. In the absence of coordinates, the headquarter’s location of the municipality or locality was used. Shapefiles for Brazil, Argentina’s provinces, and other South American countries were obtained from the IBGE (Instituto Brasileiro de Geografia e Estatística - https://www.indec.gob.ar/indec/web/Institucional-Indec-Codgeo) and Forest Gis websites (http://forest-gis.com/).
2.4 DNA extraction, PCR amplification and sequencing
The DNA for 77 specimens was obtained from one leg per individual (Supp 1). For dry samples from entomological collections, the DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, USA), while for fresh samples, it was extracted using the Chelex method (Bio-Rad, USA; Walsh et al. 1991). Two mitochondrial genes were partially amplified: COI using Barbeef and MtD9 primers (Françoso and Arias 2013; Simon et al. 1994) and CytB using mtD-26 and mtD28 primers (Simon et al. 1994). PCR amplifications for COI were performed using GoTaq® qPCR Master Mix (Promega, USA) with 1.5 µl of template DNA in a final volume of 15 µl. For CytB amplifications, 2 µl of template DNA were used in a final volume of 20 µl. PCR amplifications were carried out using a thermocycler program consisting of denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 42 °C (COI) and 38.4 °C (CytB) for 1 min and 20 s and 64 °C for 2 min, with a final extension at 64 °C for 10 min. PCR products were separated on a 0.8% agarose gel, stained with Gel Red 10,000X (Biotium, USA), and visualized under UV light. Successful PCR products were purified using ExoSAP-IT® (USB, USA) and sent for sequencing to “Centro de Facilidades de Apoio à Pesquisa” - CEFAP-USP, São Paulo, Brazil, using an automatic sequencer 3130xl Genetic Analyzer (Applied Biosystems). The DNA sequences were aligned and edited using Geneious Prime 2023.0.1 software (Drummond et al. 2011). The sequences were also analyzed using the BLAST tool against the GenBank databases (Altschul et al. 1990) and later translated to amino acids to verify contamination during amplification and possible numts (Leite 2012).
2.5 Molecular analysis
Genetic distances within and between the two Xylocopa morphotypes, nigrocincta and suspecta, described in the literature were analyzed using MEGA software version 11 (Tamura et al. 2021) with the Kimura-two-parameter (K2P; Nei and Kumar 2000) model. Bayesian inference reconstruction was performed in Mr. Bayes (Ronquist and Huelsenbeck 2003) within the Geneious software. The GTR+I model was used for both COI and CytB sequences. Markov Chain Monte Carlo sampling was used to estimate posterior distributions of parameters, including tree topology and branch lengths. Two parallel runs with four chains were performed for 1,100,000 generations, and samples from the posterior distribution were drawn every 200 generations after a burn-in of 100,000. COI and CytB sequences of Xylocopa frontalis (KC853310 and AY005275, respectively) obtained from GenBank were used as outgroups.
3 Results
3.1 Morphology and distribution
The morphological analysis revealed variations in the number of ferruginous metasomal bands, including intermediate specimens with 1 to 3 bands (Figure 1). These variations were not clearly associated with any of the nominal species examined, as described in the literature (Marchi and Alves-dos-Santos 2013; Schlindwein et al. 2003; Lucia et al. 2014). The punctuation pattern of T2 did not allow for the recognition of nigrocincta and suspecta morphotypes, as it was variable in both (Figure 2). The iridescence of the wings also exhibited significant variation in both morphotypes. While some individuals in both morphotypes displayed a strong violet iridescence throughout all the wing extension, others exhibited bluish spots, as well as other colours like metallic greenish and slightly golden in the apical region. The exclusively bluish wing iridescence was observed only in some entirely black individuals (Figure 3). Additionally, we observed a variation in the shape of the basitibial plate in both morphotypes, but no consistence to differentiate between them (Figure 4).
Variation in number of reddish metasomal terga bands present in two Xylocopa morphotypes, nigrocincta (with one or more reddish band) and suspecta (entirely black). A Metasomal terga entirely black; B one band; C, D two bands, being a little blot in T1 and entirely bands in T2 e T3; E three bands; F four bands; G five bands; H six bands. Scale bar 1 mm.
Variation in T2 metasomal punctuation of two Xylocopa morphotypes, nigrocincta and suspecta. A, D Very sparse punctuation; B, E sparse punctuation; C, F moderately dense punctuation. Scale bar 1 mm. We can observe that the bands’ colours, related to the nigrocincta (A–C; entirely black) and suspecta morphotypes (D–F, with reddish bands) are not related to punctuation pattern.
Based on the morphology of the metasomal bands’ colour and the distribution of the 226 examined specimens, three distinct morphogroups could be identified: (1) an entirely black morphogroup, predominantly distributed in the north and northeast regions of Brazil; (2) a morphogroup with four or more reddish bands, found in the southern regions of South America, specifically in the south of Brazil, north of Argentina, and south of Paraguay; and (3) an intermediate morphogroup with 1 to 3 reddish bands, distributed between the previously described morphotypes 1 and 2, mainly in the southeast of Brazil (Figure 5). The entirely black morphogroup was the most prevalent in the examined material, representing 70% (158/226) of the total, while the other two morphotypes represented 15% (34/226) each one. In contrast to the colour-based morphogroups, the pattern of T2 punctuation density did not show a clear geographic distribution pattern (Supp 2). Regions where only a single morphotype was found had a lower number of examined specimens. It is possible that all three morphs were present throughout the recorded distribution if more individuals were examined.
Morphological distribution of 226 specimens of two Xylocopa morphotypes, nigrocincta and suspecta, based on the colour of the metasomal bands. The size of the circle represents the number of registered specimens in each state or province. The smallest circumference corresponds to one individual; the intermediaries 2–5, 6–15, 16–25; and the largest to 26 individuals or more.
3.2 Molecular data
From the 77 specimens that had their DNA extracted, 58 successfully amplified the COI region with 297 bp, and from the initial 23 specimens analyzed, 19 successfully amplified the CytB region with 289 bp. The COI and CytB sequences that presented low-quality, double peaks observed in the electropherogram or stop codons when translated were excluded from the analysis. These low-quality sequences could be explained by DNA fragmentation of the entomological collection samples, numts (nuclear DNA of mitochondrial origin) and/or heteroplasmy (Françoso et al. 2015; Ricardo et al. 2020).
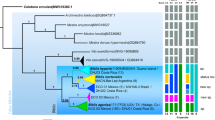
Both COI and CytB sequences presented an AT bias, with 77.1% in the COI fragment and 82.1% in the CytB fragment, as expected for insect mitochondrial DNA (Tarrio et al. 2001). Phylogenetic reconstructions based on Bayesian inference for COI and CytB fragments showed no genetic structure between the two morphotypes previously described in the literature (nigrocincta and suspecta morphotypes) nor among the three morphogroups described here based on the band colour and geographic distribution (Figure 6).
Bayesian tree based on molecular data from partial COI and CytB sequences. The samples encompass the two Xylocopa morphotypes, nigrocincta (with four or more reddish metasomal bands) and suspecta (entirely black) and individuals with an intermediate morphology (1–3 reddish band). Posterior probability support is indicated in each node. The circle colour is based in a diagnose of the number of bands on the individuals. The terminal names represent the abbreviation of the name of the morphotype (N for nigrocincta, and S for suspecta), followed by the abbreviation of Brazilian states where the sample was collected and its individual number). Xylocopa frontalis (not shown) was used as outgroup. Details of each sample are available in Supp 1.
The overall genetic distances, when considering all samples, were 1% for COI and CytB markers. Also, this same value was observed within nigrocincta and suspecta morphotypes for each marker. The distances between the nigrocincta and suspecta morphotypes were 1.17% for COI and 1.11% for CytB.
4 Discussion
The traditional morphological approach is the easiest and most common method to define species (Løken 1984; Bolton 1995; Michez and Eardley 2007). Nevertheless, morphological characters that accurately reflect taxonomic units are generally difficult to determine (Bickford et al. 2007) due to polymorphisms and phenotypic plasticity (Sigovini et al. 2016). Incorrect identifications can be frequent when cryptic or polymorphic species are present in a population and when only morphological taxonomic keys are used as identification tools (Padial et al. 2010; Ferrari and Melo 2014). In Xylocopa, the colour of different parts of the bee body, such as the integumental colouration of the metasomal terga and wings, has been extensively used as a morphological character for species recognition and delimitation (Lucia and Gonzalez 2017). Nevertheless, since these characters are variable and homoplastic in the whole genus (Lucia and Gonzalez 2017), there are limitations to this approach. In X. nigrocincta and X. suspecta, the easiness of recognizing the presence of ferruginous metasomal bands probably led Moure and Camargo (1988) not to consider the possibility that the entirely black individuals they are describing as X. suspecta could be a variation within the nominal species X. nigrocincta. Indeed, when comparing the most common morphological characters in these bees (metasomal band colour, metasomal punctuation, wing colours and apical regions of the basitibial plate), both nigrocincta and suspecta morphotypes were found to be indistinguishable, raising suspicions about the reliability of these morphological characters for species identification and delimitation in this group. Nevertheless, the metasomal band colour together with the geographic distribution revealed the presence of three distinct morphogroups: entirely black individuals in the north of their distribution, individuals with four or more reddish bands in the south, and individuals with 1 to 3 reddish bands in between the first two morphogroups (Figure 5).
Due to the morphological complexity involved in these bees, it was necessary to use an integrative approach here applied by combining the morphological review with molecular analysis (Dayrat 2005; Will et al. 2005; Pante et al. 2015) aimed at solving the limits and recognition of the taxa under study. According to the phylogenies constructed from the good-quality sequences of COI and CytB, there is no structure between the two morphotypes previously described in the literature, nigrocincta and suspecta, and consequently, neither among the three morphogroups described here. Furthermore, the genetic distance between these morphotypes is low (1.1%). Therefore, our data show no differentiation between nigrocincta and suspecta morphotypes, indicating that they belong to the same species, X. nigrocincta, and that the three morphogroups we described here represent intra-specific variations.
The same pattern, in which different morphogroups according to the geographic distribution, was found in Bombus pauloensis (Françoso et al. 2016) and in three species of Euglossa: Euglossa iopoecila, Euglossa stellfeldi, and Euglossa townsendi (Ferrari and Melo 2014). In B. pauloensis, a bee with a very similar distribution to X. nigrocincta, three different lineages were described based on morphology, geographic distribution, and genetic structure (Françoso et al. 2016). Completely black individuals are found in the north of the species’ distribution, individuals with regular yellow stripes are found in southern Brazil, and in between these two areas, in the state of São Paulo, there is a hybrid zone where all possible morphotypes can be found (black, yellow, and intermediate morphotypes) (Françoso et al. 2016). Among the Euglossa species, the green morphology is found towards the north of their geographic distribution, the blue morphology is found towards the south, and, again, an intermediate morphology was found in between these areas in at least two of the species analyzed, in Southeast Brazil (Ferrari and Melo 2014). No genetic differentiation was found in each species when comparing individuals with different morphology, showing that the different morphotypes correspond to intra-specific variations (Ferrari and Melo 2014). These results show that there is a hybrid zone in Southeast Brazil for B. pauloensis, Xylocopa morphotypes, and the at least two Euglossa species, suggesting that this area can indicate a large and common area of Pleistocene refuges with high genetic diversity. This can be extremely important for conservation, not just for these bees but for many other organisms (Françoso et al. 2016, 2018).
In conclusion, the nigrocincta and suspecta morphotypes are indistinguishable according to the molecular data and morphology, and the three different morphogroups described here represent intra-specific variation. Since the two nominal species are not distinct evolutionary lineages, a synonym between X. nigrocincta and X. suspecta is proposed here.
5 Taxonomic treatment
Xylocopa (Neoxylocopa) nigrocincta Smith, 1854
Xylocopa nigrocincta Smith, 1854: 354. Holotype: ♀, unknown, not deposited in BMNH. Holotype: South America.
Xylocopa schulthesii Dusmet & Alonso, 1924: 52; Synonymized by Hurd & Moure, 1963. Holotype: ♀, deposited in MNCN. Type locality: Brazil, Rio Grande do Sul.
Xylocopa (Neoxylocopa) nigrocincta, Hurd & Moure, 1963: 151.
Xylocopa nigrocincta jujuyensis Brèthes, 1916: 410. Synonymized by Lucia et al. (2014). Syntype: ♀, deposited in MACN. Type locality: Argentina, Jujuy.
Xylocopa (Neoxylocopa) suspecta Moure & Camargo, 1988: 209. Holotype: ♀, deposited in DZUP. Type locality: Brazil, Ribeirão Preto. New synonym.
Diagnosis
The female of this species can be distinguished from other Neoxylocopa species by the following character combinations: medium sized bees, body length 1.8–2.7 cm, usually near 2.3 cm, head width 0.6–0.8 cm; head with a frontal carina or a sharp pointed projection between antennal sockets, and without carina below lateral ocelli; scutellum angled as seen in profile; metasoma black or with ferruginous bands on terga, from one, on T1, to six T1–T6; Uniformly black or dark brown hairs, T2 with very sparse metasomal punctuation to moderately punctate and short and/or median hairs; metasomal lateral portion with dense pilosity with long hairs; dark brown wings with variable metallic luster, greenish and lightly golden in the apices or violet with blue iridescent in all extension.
Description
Female. Body length approximately 2.33 (1.8–2.7 cm); head length 0.65 (0.6–0.8 cm); head width 0.74 (0.6–0.8 cm); mesosoma length 0.7 (0.5–0.9 cm); mesosoma width 0.93 (0.6–1.3 cm); metasoma length 1.39 (1.1–1.8 cm); metasoma width 1 (0.9–1.3 cm). Integument: head entirely black; mesosoma region may be entirely black, but in some cases, there may be a slightly rusty band on the apical portion of the scutellum; metasoma region may be entirely black or with rusty bands present on the supramarginal portion of T1 to T6, with a dark band on the apical terga. Tegula black, and wings dark brown with a greenish sheen and a slight golden hue at the apices or violet with blue tones throughout their length. Pilosity: face composed of dark, simple hairs of medium length, slightly overlapping in the frontal and clypeal regions, while in the labrum region, they may have a subtly golden coloration; in the mesosoma region; in the metasoma region, dark hairs are present (except on T6, which has a slight rusty spot), with the lateral areas having a mixture of simple and plumose hairs of long length and high density. In the disc region, pilosity is simple, with short or medium length, and the overlap or non-overlap depends on the punctuation of each tergum. On T1, simple and plumose hairs are present in the disc region. T2–T4 with simple hairs of short or medium length. In the disc region of T5, the hairs are simple, medium-length, and slightly dense. On T6, the hairs are simple and long throughout their length. Punctuation: the head has moderately dense punctuation throughout its extension, except in the clypeal region, where it is very dense. Upper portion of the mesosoma is smooth. In the metasoma, punctuation varies on each tergum. On T1, it can be moderately sparse to moderately dense on the discs and dense on the sides. On T2, it can be very sparse to moderately dense on the discs and dense on the sides. T3 and T4 have moderately dense punctuation on the discs and dense on the sides. T5 and T6 have dense punctuation throughout their extension. Structure: Presence of a frontal carina and/or tubercle, without prominences above the ocelli, and the clypeus with a slight relief on the margins. Scutellum angulate and mostly black, but rusty spots may appear on the apical portion. Basitibial plate dark brown, asymmetrically bifid, with the posterior apical portion rounded and shorter than the anterior portion.
Taxonomic notes
In the search for identification keys and diagnoses of the two nominal species, there were no records found of the presence of 1, 2, or 6 rusty bands on the metasomal terga. However, during the observation of specimens, individuals with only one band or up to six metasomal rusty bands were noted, referred to in this work as intermediate forms. The wing coloration for the nominal species, X. nigrocincta and X. suspecta, was only mentioned as dark brown with a strong violet sheen and sometimes with a violet sheen near the anterior margin, respectively. However, an incredible variation was observed. Individuals were found with wings that had a strong violet sheen throughout their length, and in some cases, they had blue spots. Other individuals had wings with a metallic green sheen throughout their length, and in some cases, they had a slight golden sheen at the apices. The basitibial plate exhibited variations in the shape of the anterior apical portion.
Biological notes
The bees were collected during the day, mostly during warmer periods, from 10 am to 3 pm, between July 2018 and February 2019. The collections were made in farms with passion fruit plantations or in places containing yellow ipê trees.
Distribution
Brazil: Acre, Amazonas, Roraima, Amapá, Pará, Maranhão, Piauí, Paraíba, Pernambuco, Mato Grosso, Mato Grosso do Sul, Goiás, Distrito Federal, Tocantins, Bahia, Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul. Bolivia. Argentina: Chaco, Corrientes, Entre Rios, Formosa, Jujuy, Missiones, Santa Fé, Salta, Tucumán (Lucia et al. 2014). Uruguay and Paraguay (Moure 2022).
Data availability
GenBank accession number: OR878494-OR878537 (COI) and OR881921-OR881939 (CytB).
Code availability
Not applicable.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Bickford D, Lohman DJ, Sodhi NS et al (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Bolton B (1995) A new general catalogue of the ants of the world. Harvard University Press, Cambridge, Massachusetts
Dayrat B (2005) Towards integrative taxonomy. Biol J Lin Soc 85:407–415
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C et al (2011) Geneious v5. 4. http://www.geneious.com
Ferrari BR, Melo GAR (2014) Deceiving colors: recognition of color morphs as separate species in orchid bees is not supported by molecular evidence. Apidologie 45:641–652
Françoso E, Arias MC (2013) Cytochrome c oxidase I primers for corbiculate bees: DNA barcode and mini-barcode. Mol Ecol Resour 13:844–850
Françoso E, Gomes F, Arias MC (2015) A protocol for isolating insect mitochondrial genomes: a case study of NUMT in Melipona flavolineata (Hymenoptera: Apidae). Mitochondrial DNA 10:1–4
Françoso E, Zuntini AR, Arias MC (2018) Combining phylogeography and future climate change for conservation of Bombus morio and B. pauloensis (Hymenoptera: Apidae). J Insect Conserv 23:63–73
Françoso E, Zuntini AR, Carnaval AC, Arias MC (2016) Comparative phylogeography in the Atlantic Forest and Brazilian savannas: Pleistocene fluctuations and dispersal shape spatial patterns in two bumblebees. BMC Evol Biol 16(2):67
Giannini TC, Boff S, Cordeiro GD, Cartolano EA Jr, Veiga AK, Imperatriz-Fonseca VL, Saraiva AM (2015) Crop pollinators in Brazil: a review of reported interactions. Apidologie 46(2):209–223
Hurd PDJR, Moure JS (1963) A classification on the large carpenter bees (Xylocopini) (Hymenoptera: Apoidea). Entomology 29:1–365
Keasar T (2010) Large carpenter bees as agricultural pollinators. Psyche 927463
Leite LAR (2012) Mitochondrial pseudogenes in insect DNA barcoding: differing points of view on the same issue. Biota Neotropical 12(3)
Leys R, Cooper SJ, Schwarz MP (2002) Molecular phylogeny and historical biogeography of the large carpenter bees, genus Xylocopa (Hymenoptera: Apidae). Biol J Lin Soc 77:249–266
Løken A (1984) Scandinavian species of the genus Psithyrus Lepeletier (Hymenoptera: Apidae). Entomol Scand 23:1–45
Lucia M, Alvarez LJ, Abrahamovich AH (2014) Large carpenter bees in Argentina: systematics and notes on the biology of Xylocopa subgenus Neoxylocopa (Hymenoptera: Apidae). Zootaxa 3754:201–238
Lucia M, Gonzalez VH (2017) New species and designation of primary types in Neotropical carpenter bees of the genus Xylocopa Latreille (Hymenoptera, Apidae). J Hymenopt Res 61:31–48
Marchi P, Alves-dos-Santos I (2013) As abelhas do gênero Xylocopa Latreille (Xylocopini, Apidae) do Estado de São Paulo, Brasil. Biota Neotropical 13(2):249–269
Mawdsley JR (2018) New records and diagnostic notes on large carpenter bees (Hymenoptera: Apidae: genus Xylocopa Latreille), from the Amazon River basin of South America
Michener CD (2007) The bees of the world, 2nd edn. The Johns Hopkins University Press, Baltimore
Michez D, Eardley C (2007) Monographic revision of the bee genus Melitta Kirby 1802 (Hymenoptera: Apoidea: Melittidae). Annales de la Societe Entomologique de France (n.s.) 43:379–440
Moure JS (2022) Xylocopini Latreille, 1802. In: J. S. Moure, D. Urban, & G.A.R. Melo (Eds.), Catalogue of bees (Hymenoptera, Apoidea) in the Neotropical region - online version. Available at http://www.moure.cria.org.br/catalogue
Moure JS, Camargo JMF (1988) Uma nova espécie de Xylocopa (Neoxylocopa) do Brasil (Hymenoptera, Apoidea). Revista Brasileira De Entomologia 32(2):209–214
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Front Zool 7:16
Pante E, Schoelinck C, Puillandre N (2015) From integrative taxonomy to species description: one step beyond. Syst Biol 64:152–160
Ricardo PC, Françoso E, Arias MC (2020) Mitochondrial DNA intra-individual variation in a bumblebee species: a challenge for evolutionary studies and molecular identification. Mitochondrion 53:243–254
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Schlindwein C, Schlumpberger B, Wittmann D, Moure JS (2003) O gênero Xylocopa Latreille no Rio Grande do Sul, Brazil (Hymenoptera, Anthophoridae). Revista Brasileira De Entomologia 47(1):107–118
Sigovini M, Keppel E, Tagliapietra D (2016) Open nomenclature in the biodiversity era. Methods Ecol Evol 7(10):1217–1225
Silveira FA, Melo GAR, Almeida EAB (2002) Abelhas Brasileiras Sistemática e identificação. Composição & Arte, Belo Horizonte
Simon C, Frati F, Becknbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Smith F (1854) Catalogue of the hymenopterous insects in the collection of the British Museum, pt. Apidae 2:199–465
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Bio Evol 38:3022–3027
Tarrio R, Rodriguez-Trelles F, Ayala FJ (2001) Shared nucleotide composition biases among species and their impact on phylogenetic reconstructions of the Drosophilidae. Mol Biol Evol 18:1464–1473
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10(4):506–513
Will KW, Mishler BD, Wheeler QD (2005) The perils of DNA barcoding and the need for integrative taxonomy. Syst Biol 54:844–851
Yamamoto M, Oliveira PG, Gaglianone MC (2014) Uso sustentável e restauração da diversidade dos polinizadores autóctones na agricultura e nos ecossistemas relacionados: planos de manejo. Funbio, Rio de Janeiro
Acknowledgements
We are grateful to Marcio Oliveira (INPA), Orlando T. Silveira and Beatriz Coelho (MPEG), Kelly Ramos and Carlos Brandão (MZSP), Eduardo Almeida (RPSP), Mauro Ramalho and Maise Silva (UFBA), Maria Cristina Gaglianone (UENF), Rogério Silvestre (UFGD), Fernando Silveira (UFMG), Gustavo Graciolli (UFMS), Luciana Ianuzzi (UFPE), Marcos Antônio de Lima Bragança (UFTO) and Celso F. Martins (UFPB) for loaning specimens. Special thanks are due to Carolina Minio (UNAM-CONICET), Luiz Roberto Faria Jr. (UNILA), Hermes Schmitz (UNILA), Elaine G. Soares (UNILA) and Luiz Henrique P. Garcia (UNILA) for the help with the enlightenment discussions to analyses, elucidation of several doubts and relevant contributions and Susy Coelho for the laboratory maintenance.
Funding
We thank to UNILA for the master fellowship and aid provided for the first author. CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico (research sponsorship to MCA - 306932/2016-4) and CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (Finance Code 001). European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Fellowship to EF (grant agreement No101023351), and Fundação de Amparo `a Pesquisa do Estado de São Paulo (EF - Proc.14/25023-6).
Author information
Authors and Affiliations
Contributions
FCZ, MCA and EF designed the study; JCA and EF performed experiments and analysis; JCA, FCZ, EF and MCA interpreted the data; JCA and EF wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Field sampling and experiments were conducted in accordance with the provisions of Brazilian legislation (SISBIO ID 18669–2 and SISGEN ID A7C3F9D).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Klaus Hartfelder
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agostini, J.C., Françoso, E., Arias, M.C. et al. Morphological and molecular evidence for considering Xylocopa nigrocincta as the senior synonym of Xylocopa suspecta (Apidae: Xylocopini). Apidologie 55, 18 (2024). https://doi.org/10.1007/s13592-024-01057-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01057-9