Abstract
Honeybee Apis mellifera are considered essential pollinators in sunflower (Helianthus annuus) crops for hybrid seed production where they have to transfer pollen from male fertile (MF) to male sterile (MS) cultivars. Despite their biased preference for specific resources defined as floral constancy, it is unknown how they behave in hybrid sunflower seed crops exhibiting a noticeable dimorphism between parental lines. We studied honeybee foraging behavior in sunflower crop fields that exhibited a variable dimorphism among parental lines. Our results suggest low honeybee efficiency in terms of foraging flights between parental lines, since lower switching frequencies from MF to MS lines were observed for cultivars with increasing dimorphisms. Moreover, we also captured bees on MS capitula and at the hives to quantify the sunflower pollen grains adhered to their bee bodies with the aim to determine mechanisms responsible for the transfer of pollen between parental lines in cultivars with dimorphisms. Results show that honeybees located at the hive entrance, performing guarding and/or food receiving tasks, could act as agents facilitating pollen transfer between returning foragers and those that take off. This issue could partially compensate the low honeybee pollinating efficiency in terms of flights between parental lines.
Similar content being viewed by others
1 Introduction
During the last decades, the expansion of pollinator-dependent areas together with the decline in abundance and diversity of wild insects has led to an increasing demand of pollination services by managed insect populations (Biesmeijer et al. 2006; Oldroyd 2007; Aizen et al. 2008). The most widely used insect pollinator worldwide is the Western honeybee Apis mellifera, and despite the commercial relevance of hive products (e.g., honey and pollen), its activity as crop pollinator remains its most important economic contribution (Abrol 2012). Honeybees might not be the most efficient pollinators of most crops (Mc Gregor 1976; Delaplane and Mayer 2000; Garibaldi et al. 2013), e.g., in terms of fruit setting compared with other pollinators at an equal number of visits (Berger et al. 1988). Even though, and since they can cover large distances and are very manageable, they become well suited for pollination of large monocrops. Honeybees visit a wide range of flower types provided that the discovered resources are profitable (Visscher and Seeley 1982; Steffan-Dewenter and Kuhn 2003), but regardless of their generalist foraging strategy (von Frisch 1967), they exhibit fidelity to a particular plant species within a same foraging bout. Such behavior is known as flower constancy (Free 1963; von Frisch 1967) and implies that once the association between sensory cues and the reward has been established in a profitable floral species, bees will discriminate between flowers on the basis of the previously learnt cues and forage in a single floral species throughout a foraging boot (Menzel and Erber 1978). Floral constancy together with the ability to communicate food-related information, e.g., location of the floral resources and their chemo-sensory information to their nest mates (von Frisch 1967; Farina et al. 2005), contributes to make these eusocial insects the most effective pollinators throughout a broad spectrum of agricultural settings (Seeley 1985).
The degree to which a particular crop needs insect pollination depends on the flower morphology and the level of self-fertility exhibited by the plant together with the arrangement of flowers on the plant and neighboring plants (Klein et al. 2007). In particular, crops with separate male and female flowers (so-called imperfect flowers) are more dependent on insect pollination because they must carry the pollen from male to female flowers (Delaplane and Mayer 2000). Thus, crops of soybean, raspberry, and tomato depend moderately on insect pollination, whereas at apple, pear, alfalfa, almond, and some sunflower crops, the pollination services by bees are essential (Williams 1994). Among sunflower (Helianthus annuus) crops, those for hybrid seed production, the second most important oilseed crop worldwide, are one of the most pollination-service-dependent. They are produced by means of cytoplasmic male sterility (CMS), a technology that involves a male sterile (MS) line that needs to be pollinated with a restorer line (or male fertile plant, MF) for hybrid seed production, a mechanism that mimics natural dioecious plants (Mc Gregor 1976).
Despite the fact that the sunflower offers ample opportunity for developing new varieties through heterosis breeding (Singh et al. 1984), few studies have focused on the transfer of pollen between parental lines mediated by pollen vectors. In particular, and concerning honeybees, previous studies have reported low percentages (6.5–12.8 %) of honeybees loaded with sunflower pollen grains while visiting MS capitula (DeGrandi-Hoffman and Martin 1993). Such results suggest a low switching frequency from MF to MS parental lines, but this feature has not been studied so far.
As we described previously, once the association between sensory cues and the reward has been established in a particular flower species, honeybees will forage in that flower type throughout the foraging bout and even return to the same species in the successive foraging flights. Previous studies have described inflorescence size effects on foraging behavior and floral constancy (Ishii 2006; Gumbert and Kunze 1999), and many sunflower crops producing hybrid seeds show great morphological differences between parental lines. This scenario sets a challenge for the honeybee A. mellifera which, despite its flower constancy, is expected to fly from pollen parental lines (or MF) to seed parental lines (or MS) and cross-pollinate these crops. For this reason, among all the sensory cues learnt in a flower, in this study, we focused on the morphological ones.
Considering all the previous, in this study, we analyzed the foraging behavior of honeybees on MF plants and registered their switching frequency from MF to MS plants in four sunflower H. annuus (L.) crop fields, each producing a different hybrid seed variety and concerning a variable dimorphism between MF and MS parental lines. We hypothesized that honeybees will fly from MF to MS plants more frequently in crop fields with parental lines exhibiting morphological homogeneity.
In-hive transfer of pollen, a mechanism proposed for cross pollination of widely separated plants (Free and Durrant 1966) such as apple crops (DeGrandi-Hoffman et al. 1984; DeGrandi-Hoffman et al. 1986) and almond crops (DeGrandi-Hoffman et al. 1992), has also been suggested for pollination of sunflower crops (DeGrandi-Hoffman and Martin 1993). Considering the hive as a place to pollen transfer, we also studied the occurrence of sunflower pollen grains on the bodies of honeybees captured in the hive entrance and inside the hive. We hypothesized that individuals captured in the nest will have pollen grains on their bodies.
2 Materials and methods
2.1 Study site and experimental set up
Field and behavioral studies were performed during the sunflower blooming season in 2012 and 2013, at four crop fields containing different sunflower (H. annuus) parental lines for hybrid seed production. The hybrid seed varieties produced at crop fields 1, 2, 3, and 4 were MG360, NTC418XL, NTO1.0CL, and 8H270CLDM, respectively (DOW Agrosciences). These crop fields were located in the vicinity of Hilario Ascasubi (39° 22′ 0″ S, 62° 39′ 0″ W) and Pedro Luro (39° 30′ 0″ S, 62° 41′ 0″ W), province of Buenos Aires, Argentina, and managed by DOW Agrosciences. The arrangement of the male fertile (MF) and male sterile (MS) lines in the four agricultural settings consisted of two MF lines every eight MS ones, alternated in this proportion throughout the field width.
In the agriculture settings considered in this study, MF lines bloomed earlier than MS lines. As for the blooming period, observations were carried out at the beginning of the MS flowering period, which occurs during the MF line full bloom. We chose this period to avoid the flowering of the MS inflorescence central area. Many studies account for a low seed in it, and it is unknown whether factors influencing low seed set might also affect honeybee foraging behavior (Connor and Hall 1997). As for the MF flowering period, the full bloom ensured the availability of fresh pollen throughout the study.
Colonies of European honeybees (A. mellifera L.) with a mated queen, three or four frames of capped brood, food reserves, and about 20,000 individuals were located around the fields mentioned above. Ten-frame Langstroth hives were set in groups of 10 to 15 hives each, so that the colony density achieved was of two to five hives per hectare in all plots.
2.2 Honeybee foraging behavior on sunflower inflorescences
We studied honeybee foraging behavior in two fields (crop fields 1 and 2; henceforth, CF1 and CF2) during January 2012 and in the other two (crop fields 3 and 4; henceforth, CF3 and CF4) during January 2013. Honeybees collect pollen more conspicuously in the morning. However, to include both pollen and nectar foragers, we made the observations of the honeybees’ behavior on the MF inflorescences between 8:00 and 6:30 pm in both years. Records consisted of monitoring individual honeybees from the moment they landed on a MF sunflower capitulum and while they moved in between plants of the same (MF) or across the MS cultivar. For each bee, we registered the sequential foraging visits, recording the parental line visited and the time spent on each capitulum, until the observer lost sight of the focus bee.
To calculate the percentage of bees that showed constancy on MF lines in every field, we considered the following: number of bees constantly foraging on MF plants / [(number of bees constantly foraging on MF) + (number of bees switching from MF to MS)] * 100. Bees exhibiting cultivar constancy (i.e., bees exclusively foraging on MF plants) were also considered to calculate foraging mean time per inflorescence and to estimate the number of inflorescences visited per bee in the course of a foraging trip at each field.
Additionally, and with descriptive purposes, we registered the mentioned variables for honeybees foraging on MS lines regardless the distance to MF lines.
2.3 Sunflower morphology and palynology
In each of the four mentioned crop fields, all of which produced a different hybrid seed, we measured the height and capitulum diameter (cm) of 30 MS and 30 MF plants and collected a pollen sample from the latter to assess its grain size. The capitulum diameter measurement comprised the whole disc, from a border to the other crossing the center of the capitula. For pollen collection, we shook the anthers of every pollen parental over a plastic jar (a different jar per pollen parental), and we closed the container and maintained the samples in a refrigerator for further analysis. Once at the laboratory, we put a drop of distilled water on a slide, poured a small sample of pollen on it, and stained it with Lugol solution. After adding the coverslip, we recorded the pollen size (μm) of 30 grains per pollen bearing parental line (Labomed CXR III microscope).
2.4 Pollen grains adhered to the bodies of honeybees
In January 2013, we registered the occurrence of sunflower pollen grains adhered to the bodies of bees captured both at the entrance and inside A. mellifera hives that pollinated two of the crop fields included for previous analysis (CF3 and CF4). At the hive, we captured three groups of bees: One group, defined as incoming bees, consisted of workers trapped at the entrance platform just after landing. The second one included bees standing at the hive entrance (entrance bees) that did not exhibit any conspicuous behavior to take off. A third group of bees was captured inside the hive. To capture the last group, we removed the hive roof and took workers that were standing near the brood area (henceforth, in-hive bees). Per plot, we caught 10 bees of each group (incoming, entrance, and in-hive) in 10 different hives. Additionally, and with descriptive purposes, we caught 30 foraging bees on 30 randomly chosen MS plants (a bee per MS capitulum). The samples at the MS plants and at the hives were carried out at the beginning of the MS flowering period, which occurs during the MF line full bloom. All the captured bees were frozen singly.
At the laboratory, we poured a drop of distilled water on a slide and rolled a single bee on it, so that its entire body surface would come into contact with the liquid. We stained the sample with Lugol solution, added the coverslip, and registered the presence/absence of sunflower pollen grains (Labomed CXR III microscope).
2.5 Statistics
We analyze floral constancy with a chi-square homogeneity test for constant and inconstant foragers, considering the four pollen parent producers (Sokal and Rohlf 1995).
Mean time per inflorescence and the number of inflorescences visited were analyzed with a one-way analysis of variance (ANOVA) considering crop field as a four-level factor, followed by a Tukey test (Zar 1999). To meet the assumptions of normality and homogeneity of variances, we used log10 to transform the data accounting for number of inflorescences visited in MF cultivar, and we re-sampled it.
Capitulum diameter and height of plant of both MS and MF inflorescences were analyzed using a two-way analysis of variance (ANOVA; (Sokal and Rohlf 1995) with cultivar as a two-level factor (MS and MF) and crop field as a four-level factor (CF1, CF2, CF3, and CF4). To meet the assumptions of normality and homogeneity of variances, we used log10 to transform the data. When we detected statistical differences in the interaction between factors, we computed multiple comparisons of interaction using the corresponding error (Zar 1999).
Pollen grain size was analyzed with a one-way analysis of variance (ANOVA) with crop field as a four-level factor, followed by a Tukey test (Zar 1999).
The percentage of bees with pollen on their bodies captured both at the hive entrance and inside the hive was analyzed with a two-way repeated measure analysis of variance (ANOVA-RM) with crop field as a two-level factor and group of bees as a three-level factor. Since we detected no statistical differences in the crop field * group of bees interaction factor, we computed principal effects (Zar 1999). The percentage of bees with pollen on their bodies, for bees captured on MS capitula, was studied with a chi-square homogeneity test (Sokal and Rohlf 1995).
3 Results
3.1 Honeybee foraging behavior
We observed a noticeable persistence to forage on MF capitula along the successive visits. The maximum switching frequency from MF to MS parental lines, the more relevant behavior in terms of cross-pollination, was 15 %, and it was observed at crop field 1 (CF1). We rejected homogeneity of constant and not constant foraging behavior among all pollen parental lines (chi-square homogeneity test, F = 9.673, d.f. = 3; P = 0.0216), but not when we excluded CF1 from the analysis (chi-square homogeneity test, F = 5.579, d.f. = 3; P = 0.1340; Figure 1). This analysis proves that honeybees behave differently in crop field 1 concerning switching frequency from MF to MS parental line. It is important to note that, even if we did not analyze it, most of the bees foraged for nectar in this cultivar both in the morning and during the afternoon, and the occurrence of individuals with pollen on their hind legs (corbicula) was similar throughout the day.
Honeybee floral constancy on the male fertile (MF) plants at different hybrid seed producers. The percentage of bees constantly foraging on the MF parental is represented with gray (constantly). The black bars represent the percentage of bees switching from MF to male sterile (MS) plants (not constantly). The numbers in the columns indicate the number of honeybees whose foraging behavior was studied in each crop field. Different letters indicate statistical differences (P < 0.05; chi-square homogeneity test for switching frequency). CF1 crop field 1, CF2 crop field 2, CF3 crop field 3, CF4 crop field 4.
The time spent onto the inflorescence by honeybees that showed MF constancy exhibited significant differences between pollen parental lines. Bees spent the longest foraging time on MF plants in CF1 (one-way ANOVA, F = 6.56; d.f = 3, P < 0.001; Tukey Test, P < 0.05; Figure 2). Concerning mean (±SE) number of inflorescences visited per constant bee, we also found significant differences, i.e., honeybees visited more plants at crop field 3 (for CF1, 4.45 ± 0.61; for CF2, 4.35 ± 0.63; for CF3, 8.85 ± 1.47; for CF4, 5.3 ± 0.79; one-way ANOVA, F = 5.98; d.f. = 3; P < 0.01; Tukey Test, P < 0.05).
Time spent by honeybees on each MF sunflower capitulum at different hybrid seed producers (mean ± SE). Observations began once a honeybee landed on a MF capitulum and continued along its successive visits to different inflorescences, until observers lost sight of the focal bees. The numbers in the columns indicate the number of honeybees whose foraging behavior was studied in each crop field. Different letters indicate statistical differences (P < 0.05; Tukey comparison, one-way ANOVA). CF1 crop field 1, CF2 crop field 2, CF3 crop field 3, CF4 crop field 4.
Honeybees foraging on male sterile capitula exhibited no statistical differences between crop fields in their switching frequency from MS to MF lines. As for constant bee behavior, there were no statistical differences between crop fields neither in the time they spent on each plant (mean time in seconds on each MS inflorescence visited, for CF1, 60.3 ± 17.56; for CF2, 73.4 ± 15.94; for CF3, 85.4 ± 9.39; for CF4, 56.7 ± 11.11; one-way ANOVA, F = 1.46; d.f. = 3, P = 0.2297) nor in the number of MS plants visited (mean number of inflorescences visited, for CF1, 5.1 ± 0.92; for CF2, 4.2 ± 0.38; for CF3, 4.9 ± 0.51; for CF4, 4.8 ± 0.46; one-way ANOVA, F = 0.27; d.f. = 3, P = 0.850).
3.2 Sunflower and pollen morphology
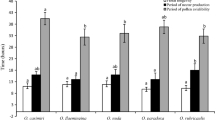
Striking differences between MS and MF cultivars were found in some of the studied crop fields (Figure 3). Specially, sharp differences were observed in plant height between parental lines at crop fields 3 and 4 (two-way ANOVA, interaction factor cultivar * crop field: F = 37.5; d.f. = 3, P < 0.0001; interaction Tukey Test, P < 0.05; Figure 3a). As for capitulum diameter, we obtained significant differences between cultivars in all fields except in the crop field 1 (two-way ANOVA, interaction factor cultivar * crop field, F = 18.87; d.f. = 3, P < 0.0001; interaction Tukey test, P < 0.05; Figure 3b). With regards to pollen grain size, we found heterogeneity among pollen cultivars, and the biggest pollen grains belonged to pollen parental of cop field 2 and the smallest ones to pollen parental of crop field 4 (one-way ANOVA, F = 52.95; d.f. = 3, P < 0.0001; Tukey Test, P < 0.05; Figure 3c).
Morphological variables of sunflower inflorescences. a Plant height, in centimeter (mean ± SE), and b capitulum diameter, in centimeter (mean ± SE) of male sterile (MS) and male fertile (MF) plants of four crop fields. c Pollen size (MF), in micrometer (mean ± SE; N = 30). Different letters indicate statistical differences (P < 0.05; Tukey comparisons cultivar * hybrid interaction factor, two-way ANOVA for A and B; Tukey comparison, one-way ANOVA for (c)). CF1 crop field 1, CF2 crop field 2, CF3 crop field 3, CF4, crop field 4.
3.3 Pollen grains on the honeybees
The percentage of bees with pollen on their bodies captured on MS inflorescences was significantly different between crop fields 3 and 4 (chi-square homogeneity test, F = 14.831; d.f. = 1; P = 0.0001; Figure 4a).
Honeybees with sunflower pollen on their bodies. a Foragers captured on male sterile (MS) inflorescences with sunflower pollen on their bodies (in percentage) in both crop fields, CF3 and CF4. b Honeybees captured in hives located in the surrounding of CF3 and CF4 with sunflower pollen on their body (in percentage). The categories of bees captured were as follows: in-hive bees, incoming bees, and bees at the hive entrance (entrance bees). N in (a) indicates the number of bees analyzed and in (b) number of colonies. a ***P < 0.001, two-way ANOVA-RM; b ***P < 0.001, chi-square homogeneity test.
The percentage of bees captured at hive entrance and inside the hive, with pollen on their bodies, was different among all groups. Interaction factor crop field * group of bees was not significant (F = 2.18; d.f. = 2; P = 0.128), but the group of bees factor showed significant differences (two-way ANOVA-RM, F = 35.83; d.f. = 2, P < 0.0001; Tukey test; P < 0.05; Figure 4b).
It is interesting to note that in CF3, the percentage of foraging honeybees captured on MS inflorescences with sunflower pollen grains on their bodies was similar to the one of incoming bees. For CF4, this percentage was higher and analogous to the bee group remaining at the hive entrance defined as entrance bees (Figure 4a, b).
4 Discussion
The most striking result of this study is that the honeybee exhibits a noticeable floral constancy on male fertile plants in this commercial crop species that depends on its pollination service. Summarizing, these results show that the higher the morphological differences between parental lines, the lower the percentage of honeybees that switch from MF to MS inflorescences.
Honeybees showed the highest switching frequencies from MF to MS plants when parental lines did not differ morphologically (crop field 1). In this context, bees behaved similarly on both cultivars in terms of the time spent on the inflorescences and the number of plants visited. A lower switching frequency was found in CF2, and MS plants were similar to MF ones in height but with wider capitula. The greater number of flowers available in the wider capitula could explain why bees spent two times the amount of time on MS than on MF plants in crop and agrees with previous observations of bumblebees foraging on large inflorescences (Ishii 2006). Mean time per MF inflorescence did not differ between CF1 and CF2 suggesting that it would depend on the availability of reward. The number of inflorescences visited in both parental lines was similar in this case. For the cases of crop fields 3 and 4, we found a very low percentage or no bees flying from MF to MS lines (2 and 0 %, respectively), and cultivars were strikingly different in these crops both in terms of the plant height and the capitula diameter. The time spent was distinctly different between parental lines, and in one case (CF3), bees visited two times the number of MS than MF plants.
Present results suggest that the sensory cues considered in this study (inflorescence morphology) at least represent the set of cues honeybees recognize during foraging when considering MS and MF capitula as unique or different floral patches. Our observations suggest that heterogeneity between parental lines impairs pollination efficiency in this agricultural ecosystem as a result of the reduced frequency of flights between parental lines. The crop arrangement might also contribute to the constant foraging behavior observed. Previous studies described (Marden and Waddington 1981) that constant foraging occurs with a high probability when bees encounter a match with its current flower target within a short time window (3 s) of flight.
It is important to note that even in the crop fields with parental morphological homogeneity, as it was observed in CF1, we registered a low percentage of honeybees flying across parental lines: 15 % was the maximum value recorded, consistent with previous observations (DeGrandi-Hoffman and Martin 1993). Previous reports state that floral constancy increases with increasing differences between floral characteristics such as odor, color, and shape (Waser 1986). It is possible then that differences between MS an MF lines in terms of sensory cues other than visual clues, such as pollen odors and floral scents, combined with differences in nectar quality and availability may also be influencing honeybee behavior and promoting flower constancy. Moreover, it was observed that pollinators tend to be inconstant when flowers vary only in one trait (Waser 1986; Goulson and Wright 1998).
The presence of sunflower pollen grains on the bodies of the honeybees captured in the hives suggests that body contacts between nest mates could allow the distribution of pollen grains among hive mates and contribute to cross-pollinate this crop. This transfer of pollen is particularly obvious since a high percentage of honeybees captured at the hive entrance exhibited pollen adhered to their bodies.
Differences between CF3 and CF4 in terms of the percentage of bees with pollen grains on their bodies were found both in hives and on MS capitula, probably as a result of pollen grain size. Sunflower pollen grains have an ornamented exine and frequently form clumps of five or more grains (Seiler 1997). Adhesion forces between rough particles within the range of sizes of the pollen grains observed in CF3 and CF4 increases in proportion to the diameter of the particles (Kendall and Stainton 2001). If clump formation were more frequent among CF3 pollen grains, this could impair pollen transfer from anthers to insects by means of electrostatic forces (Corbet et al. 1982). A lower pollen transfer to the bodies of bees foraging on MF plants in CF3 could account for the lower percentage of bees captured on MS inflorescences with pollen on their bodies for the biggest grain type.
Previous studies concerning sunflower pollen viability stuck on the bodies of honeybees reported a 45 and 9–10 % CMS line seed setting of 12- and 36-h-old grains, respectively (Shahzad and Rashid 2006). Cross-pollinating by honeybees might therefore concern not only insects flying across lines but also other means of pollen transference such as body contacts in their colonies. The percentage of hive bees we recorded with pollen on their bodies as well as those reported by previous studies supports this idea (DeGrandi-Hoffman and Martin 1993).When foragers return to the nest, different social interactions occur between nest mates and incoming bees (Balbuena et al. 2012). Honeybees located at the hive entrance involved in guarding, fanning, or foraging tasks would have a crucial role as pollen vectors between returning and departing foragers (Lindauer 1952; Pacheco and Breed 2008). As a result, deliberate body contacts (plus other incidental ones) propagate the pollen adhered to the body hairs (Free and Williams 1972) and amplify its transference from MF to MS lines. Thus, despite that honeybees perform low-efficiency pollen transfer in terms of flights between parental lines in these agricultural settings, social interactions among nestmates partially compensate this deficit.
It is worth to reward that we focused on MF rows data. The addition of seed set data in MS rows at various distances from the MF would have been useful for determining if the observations on floral constancy and pollen transfer among bees in the hive actually affected seed set (DeGrandi-Hoffman and Martin 1993), a factor that should be taken into consideration in future studies.
Provided floral constancy, wider MF capitula would increase pollen availability, and in this sense, this one and other alternatives such as devices to maximize the transfer of pollen at the hive entrance should be explored to improve crop yield in this agricultural settings (Hatjina et al. 1988). Related to this issue and as an implication of these results, seed producers should consider not only botanical characters to obtain specific characteristics of the new hybrids but also the cognitive abilities of the essential pollinator of these commercial crops.
References
Abrol, D. P. (2012) Pollination biology. Biodiversity conservation and agricultural production, Springer
Aizen, M.A., Garibaldi, L.A., Cunningham, S.A., Klein, A.M. (2008) Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18, 1572–1575
Balbuena, M.S., Molinas, J., Farina, W.M. (2012) Honeybee recruitment to scented food sources: correlations between in-hive social interactions and foraging decisions. Behav. Ecol. Sociobiol. 66, 445–452
Berger, L.A., Vaissière, B.E., Moffett, J.O., Merritt, S.J. (1988) Bombus spp. (Hymenoptera: Apidae) as pollinators of male-sterile upland cotton on the Texas high plains. Environ. Entomol. 17, 789–794
Biesmeijer, J.C., Roberts, S.P.M., Reemer, M., Ohlemüller, R., Edwards, M., Peeters, T., Schaffers, A.P., Potts, S.G., Kleukers, R., Thomas, C.D., Settele, J., Kunin, W.E. (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354
Corbet, S.A., Beament, J., Eisikowitch, D. (1982) Are electrostatic forces involved in pollen transfer? Plant. Cell Envir. 5, 125–129
Connor D.J., Hall A.J. (1997) Sunflower physiology. in sunflower technology and production (ed. A.A. Schneiter), pp. 113–182. ASA, CSSA and SSSA, Madison, WI, USA
DeGrandi-Hoffman, G., Hoopingarner, R., Baker, K. (1984) Pollen transfer in apple orchards tree-to-tree or bee-to-bee? Bee Word. 65(3), 126–133
DeGrandi-Hoffman, G., Hoopingarner, R., Klomparens, K. (1986) Influence of honey bee (Hymenoptera: Apidae) in-hive pollen transfer on cross-pollination and fruit set in apple. Environ. Entomol. 15(3), 723–725
DeGrandi-Hoffman, G., Thorp, R., Loper, G., Eisikowitch, D. (1992) Identification and distribution of cross-pollinating honey bees on almonds. J. Appl. Ecol. 29, 238–246
DeGrandi-Hoffman, G., Martin, J.H. (1993) The size and distribution of the honey bee (Apis mellifera L.) cross-pollinating population on male-sterile sunflowers (Helianthus annuus L.). J. Apic. Res. 32, 135–142
Delaplane, K.S., Mayer, D.F. (2000) Crop pollination by Bees. CABI Publishing, New York
Farina, W.M., Grüter, C., Díaz, P.C. (2005) Social learning of floral odours inside the honeybee hive. Proc. R. Soc. Lond. B. 272, 1923–1928
Free, J.B. (1963) The flower constancy of honeybees. J. Anim. Ecol. 32, 119–131
Free, J.B., Durrant, A.J. (1966) The transport of pollen by honeybees from one foraging trip to the next. J. Hortic. Sci. 41, 87–89
Free, J.B., Williams, I.H. (1972) The transport of pollen on the boby hairs of honeybees (Apis mellifera) and bumblebees (Bombus spp. L.). J. Appl. Ecol 9(2), 609–615
Garibaldi, L.A., Steffan-Dewenter, I., Winfree, R., Aizen, M.A., Bommarco, R., et al. (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 339, 1608–1611
Goulson, D., Wright, N.P. (1998) Flower constancy in the hoverflies Episyrphus balteatus (Degeer) and Syrphus ribesii (L.)(Syrphidae). Behav. Ecol 9(3), 213–219
Gumbert, A., Kunze, J. (1999) Inflorescence height affects visitation behavior of bees –a case study of an aquatic plant community in Bolivia. Biotropica 31(3), 466–477
Hatjina, F., Free, J.B., Paxton, R.J. (1988) Hive-entrance pollen transfer devices to increase the cross-pollination of honeybee. I. Examination of six materials. J. Apic. Res. 37(4), 231–237
Ishii, H.S. (2006) Floral display size influences subsequent plant choice by bumble bees. Funct. Ecol. 20(2), 233–238
Kendall, K., Stainton, C. (2001) Adhesion and aggregation of fine particles. Powder Technol. 121, 223–229
Klein, A.M., Vaissière, B.E., Cane, J.H, Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 274(1608), 303–313
Lindauer, M. (1952) Ein beitrag zur frage der Arbeitsteilung im Bienenstaat. Zeit. vergl. Physiol. 34, 299–345
Marden, J.H., Waddington, K.D. (1981) Floral choices by honeybees in relation to the relative distances to flowers. Physiol. Entomol. 6(4):431–435
Mc Gregor, M.C. (1976) Insect pollination of cultivated crop plants. US Department of Agriculture, Washington D.C
Menzel, R., Erber, J. (1978) Learning and memory in bees. Sci. Am. 239, 80–87
Oldroyd, B.P. (2007) What’s killing american honey bees? PLoS Biol. 5(6), 1195–1199
Pacheco, J., Breed, M.D. (2008) Sucrose-response thresholds and the expression of behavioural tasks by middle-aged honeybee workers. Anim. Behav. 76, 1641–1646
Seiler, G.J. (1997) Anatomy and morphology of sunflower. In: Sunflower technology and production. Agronomy Monograph no. 35.Madison, WI: American Society of Agronomy: Schneiter A.A., ed. 67–111
Seeley, T.D. (1985) Honeybee ecology. Princeton University Press, Princeton
Shahzad, M.A., Rashid, M. (2006) Determination of pollen viability and role of honey bees Apis cerana in the pollination of sunflower CMS lines in isolated tunnels. Pak. Entomol 28(2), 69–72
Singh, S.B., Labana, K.S., Virk, D.S. (1984) Heterosis in variety x inbred crosses of sunflower. Crop Improv. 11, 35–38
Sokal, R.R., Rohlf, F.J. (1995) Biometry: the principles and practice of statistics in biological research, New York. p. 715
Steffan-Dewenter, I., Kuhn, A. (2003) Honeybee foraging in differentially structured landscapes. Proc. R. Soc. Lond. B. 270(1515), 569–575
Visscher, P.K., Seeley, T.D. (1982) Foraging strategy of honeybee colonies in a temperate decidous forest. Ecology 63(6), 1790–1801
von Frisch, K. (1967) The dance language and orientation in honey bees. Harvard University Press, Cambridge
Waser, N.M. (1986) Flower constancy: definition, cause and measurement. Am. Nat. 63(6), 593–603
Williams, I.H. (1994) The dependence of crop production within the European Union on pollination by honey bees. Agric. Zool. Rev. 6, 229–257
Zar, J.H. (1999) Biostatistical Analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgments
Authors are grateful to A. Arenas and C. Mengoni Goñalons for their support during the measuring period and two anonymous referees for their valuable comments and suggestions on an earlier version of this manuscript. We also thank R. Sardi, H. Minnucci, and DOW Agrosciences SA for their selfless support at the agricultural setting. This study was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013 1060), University of Buenos Aires (UBACyT 20020130100185BA), and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 112-200801-00150) to WMF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript Editor: Stan Schneider
Constance florale de l’abeille et son efficacité pollinisatrice dans les cultures de tournesol ( Helianthus annuus ) pour la production de graines hybrides
Apis mellifera / tournesol / pollinisation des cultures / transfert de pollen
Blütenstetigkeit und Bestäubungseffektivität von Honigbienen bei der Produktion von Hybridsamen von Sonnenblumen ( Helianthus annuus )
Apis mellifera / Sonnenblumen / Bestäubung/ Blütenstetigkeit / Pollentransfer
Rights and permissions
About this article
Cite this article
Susic Martin, C., Farina, W.M. Honeybee floral constancy and pollination efficiency in sunflower (Helianthus annuus) crops for hybrid seed production. Apidologie 47, 161–170 (2016). https://doi.org/10.1007/s13592-015-0384-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-015-0384-8