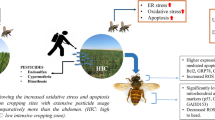
Abstract
Pesticides have been cited as one of the major drivers of pollinator loss. However, little is known about pesticide impacts on natural populations of native honey bee species. This study looked into the effect of pesticides with respect to oxidative stress in the laboratory and in field populations of two native Indian honey bee species (Apis dorsata and A. cerana) by examining a combination of biomarkers, e.g., superoxide dismutase, catalase and xanthine oxidase. A significant upregulation of all three biomarkers was observed in both treated individuals in laboratory experiments and field populations sampled from a pesticide use gradient. This study reports, for the first time, an increase in expression of xanthine oxidase in an invertebrate system (honey bees) exposed to pesticides.








Similar content being viewed by others
REFERENCES
Abrol, D.P. (2011) Foraging. In: Hepburn, H.R., Radloff, S.E. (eds.) Honeybees of Asia, pp. 257–292. Springer, New York
Aebi, H. (1984) Catalase, in : Packer, L. (Ed.), Methods in Enzymology. Academic Press, Orlando, pp. 121–126. Agricultural census, Government of India (http://agcensus.nic.in/).
Ahmad, S., Duval, D.L., Weinhold, L.C., Pardini, R.S. (1991) Cabbage looper antioxidant enzymes: tissue specificity. Insect Biochem. 21(5), 563–572
Akhgari, M., Abdollahi, M., Kebryaeezadeh, A., Hosseini, R., Sabzevari, O. (2003) Biochemical evidence for free radicalinduced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 22, 205–211
Anderson, A.D., Patton, R.L. (1955) In vitro studies of uric acid synthesis in insects. J. Exp. Zool. 128(3), 443–451
Aranda, R., Doménech, E., Rus, A.D., Real, J.T., Sastre, J., Viña, J., Pallardó, F.V. (2007) Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic. Res. 41(11), 1195–1200
Barbeta, C., Lino-Neto, T., Piques, M.C., Sousa, M.F., Tavares, R.M., Pais, M.S. (2004) Identification of Zantedeschia aethiopica Cat1 and Cat2 catalase genes and their expression analysis during spathe senescence and regreening. Plant Sci. 167, 889–898
Archer, C.R., Pirk, C.W.W., Wright, G.A., Nicolson, S.W. (2014) Nutrition affects survival in African honeybees exposed to interacting stressors. Funct. Ecol. . doi:10.1111/1365-2435.12226
Bouayed, J., Bohn, T. (2010) Exogenous antioxidants—double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative. Med. Cell Longev. 3, 228–237
Badiou-Bénéteau, A., Carvalho, S.M., Brunet, J.-L., Carvalho, G.A., Buleté, A., Giroud, B., Belzunces, L.P. (2012) Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 82, 22–31
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Brittain, C.A., Vighi, M., Bommarco, R., Settele, J., Potts, S.G. (2010) Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 11, 106–115
Choudhary, A., Sharma, D.C., Badiyala, A. (2009) Relative safety of some pesticides against honey bees, Apis cerana cerana Fab. and Apis mellifera L. on mustard (Brassica Juncea L. Czern). Pestic. Res. J. 21(1), 67–70
Claudianos, C., Ranson, H., Johnson, R.M., Biswas, S., Schuler, M.A., Berenbaum, M.R., Eyereisen, R., Oakeshott, J.G. (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 15(5), 615–636
Conners, D.E. (2004) Biomarkers of oxidative stress in freshwater clams (Corbicula fluminea), as mechanistic tools to evaluate the impairment of stream ecosystem health by lawn care pesticides. Ph. D. Thesis, Graduate Faculty, University of Georgia, Georgia.
Cresswell, J.E., Laycock, I. (2012) Towards the comparative ecotoxicology of bees: the response-response relationship. Julius-Kuhn-Archiv 437
Czarniewska, E., Kasprzyk, A., Ziemnicki, K. (2003) Effect of paraquat and metoxychlor on antioxidant enzymes in frog Rana esculenta L liver. Biol. Lett. 40(2), 125–133
Decourtye, A., Armengaud, C., Renou, M., Devillers, J., Cluzeau, S. (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 48, 242–250
Decourtye, A., Armengaud, C., Renou, M., Devillers, J., Cluzeau, S. (2004) Imidacloprid impairs memory and brain metabolism in the honeybee (Apismellifera L.). Pestic. Biochem. Phys. 78, 83–92
Desneux, N., Decourtye, A., Delpuech, J.M. (2007) The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106
Devillers, J., Pham-Delegue, M.H., Decourtye, A., Budzinski, H., Cluzeau, S., Maurin, G. (2002) Structure-toxicity modeling of pesticides to honey bees. Environ. Res. 13(7–8), 641–648
Dhaliwai, H.S., Sharma, P.L. (1974) Foraging range of the Indian honeybee. J. Apic. Res. 13, 137–141
Dyer, F.C., Seeley, T.D. (1991) Dance dialects and foraging range in three Asian honey bee species. Behav. Ecol. Sociobiol. 28, 227–233
Felton, G.W., Summers, C.B. (1995) Antioxidant systems in insects. Arch. Insect Bioch. Physiol. 29(2), 187–197
Fridovich, I. (1982) Superoxide dismutase in biology and medicine. In: Autor, A.P. (ed.) Pathology of Oxygen, pp. 1–19. Academic, New York
Fujiyuki, T., Matsuzaka, E., Nakaoka, T., Takeuchi, H., Wakamoto, A., Ohka, S., Sekimizu, K., Nomoto, A., Kubo, T. (2009) Distribution of Kakugo virus and its effects on the gene expression profile in the brain of the worker honeybee Apis mellifera L. J. Virol. 83(22), 11560
George, P.J.E., Ambrose, D.P. (2004) Toxic effects of insecticides in the histomorphology of alimentary canal, testis and ovary in a reduviid Rhynocoris kumarii Ambrose and Livingstone (Hemiptera: Reduviidae). J. Adv. Zool. 25, 46–50
Gilbert, M.D., Wilkinson, C.F. (1974) Microsomal oxidases in honey bee, Apis mellifera (L.). Pestic. Biochem. Physiol. 4(1), 56–66
Halliwell, B. (2007) Biochemistry of oxidative stress. Biochem. Soc. Trans. 35(Pt 5), 1147–1150
Han, P., Niu, C.-Y., Lei, C.-L., Cui, J.-J., Desneux, N. (2010) Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 19(8), 1612–1619
Hardstone, M.C., Scott, J.G. (2010) Is Apis mellifera more sensitive to insecticides than other insects. Pest Manag. Sci. 66(11), 1171–1180
Harrison, R. (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 33(6), 774–797
Hayashi, Y. (1961) Properties of xanthine dehydrogenase in the silkworm, Bombyx mori L. Nature 192, 756–757
Henry, N., Béguin, M., Requier, F., Rollin, O., Odoux, J.-F., Aupinel, P., Aptel, J., Tchamitchian, S., Decourtye, A. (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336(6079), 348–350
Hille, R., Nishino, T. (1995) Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 9(11), 995–1003
Idris, A.B., Grafius, E. (1993) Pesticides affect immature stages of Diadegma insulare (Hymenoptera, Ichneumonidae) and its host, the diamondback moth (Lepidoptera, Plutellidae). J. Econ. Entomol. 86, 1203–1212
Irzykiewicz, H. (1955) Xanthine oxidase of the clothes moth, Tineola bisselliella, and some other insects. Aust. J. Biol. Sci. 8, 369–377
Joanisse, D.R., Storey, K.B. (1996) Oxidative stress and antioxidants in overwintering larvae of cold-hardy goldenrod gall insects. J. Exp. Biol. 199(Pt 7), 1483–1491
Johnson, R.M., Ellis, M.D., Mullin, C., Frazier, M. (2010) Pesticides and honey bee toxicity – USA. Apidologie 41(3), 312–331
Khessiba, A., Roméo, M., Aïssa, P. (2005) Effects of some environmental parameters on catalase activity measured in the mussel (Mytilus galloprovincialis) exposed to lindane. Environ. Pollut. 133(2), 275–281
Klein, J.P., Moeschberger, M.L. (2003) Survival Analysis. Techniques for Censored and Truncated Data, 2nd edn. Springer Publishers, New York
Koeniger, N., Vorwohl, G. (1979) Competition for food among four sympatric species of Apini in Sri Lanka (A. dorsata, A. cerana, A. florea and Trigona iridipennis). J. Apic. Res. 18, 95–109
Koetz, A.H. (2013) Ecology, behaviour and control of Apis cerana with a focus on relevance to the Australian incursion. Insects 4, 558–592
Krupke, C.H., Hunt, G.J., Eitzer, B.D., Andino, G., Given, K. (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7(1), e29268
Landa, V., Sula, J., Marec, F., Matha, V., Soldan, T. (1991) Methods for Assessing Exposure of insects. In: Tardiffand, R.G., Goldstei, B. (eds.) Human and Non-Human Biota, pp. 249–266. John Wiley and Sons Ltd., New York
Mamidala, P., Jones, S.C., Mittapalli, O. (2011) Metabolic resistance in bed bugs. Insects 2(1), 36–48
Marklund, S., Marklund, G. (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47(3), 469–474
McCord, J.M., Fridovich, I. (1969) Superoxide dismutase: an enzymic function for erythrocuprein (heinocuprein). J. Biol. Chem. 244(22), 6049–6055
Medina, P., Budia, F., Del Estal, P., Vinuela, E. (2004) Influence of azadirachtin, a botanical insecticide, on Chrysoperla carnea (Stephens) reproduction: toxicity and ultrastructural approach. J. Econ. Entomol. 97, 43–50
Meneshian, A., Bulkley, G.B. (2002) The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 9(3), 161–175
Mueller, S., Riedel, H.-D., Stremmel, W. (1997) Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 90(12), 4973–4978
Mullin, C.A., Frazier, M., Frazier, J.L., Ashcraft, S., Simonds, R., vanEngelsdorp, D., Pettis, J.S. (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5(3), e9754
Nishida, Y. (2011) The chemical process of oxidative stress by copper (II) and iron (III) ions in several neurodegenerative disorders. Monatsh. Chem. 142, 375–384
Nishino, T., Okamoto, K., Kawaguchi, Y., Hori, H., Matsumura, T., Eger, B.T., Pai, E.F., Nishino, T. (2005) Mechanism of the conversion of xanthine dehydrogenase to xanthine oxidase: identification of the two cysteine disulfide bonds and crystal structure of a non-convertible rat liver xanthine dehydrogenase mutant. J. Biol. Chem. 280(26), 24888–24894
Osborne, J.L. (2012) Bumble bees and pesticides. Nature 491(7422), 43–45
Palmer, M.J., Moffat, C., Saranzewa, N., Harvey, J., Wright, G.A., Connolly, C.N. (2013) Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634
Partap, U. (2011) The Pollination Role of Honeybees. In: Hepburn, H.R., Radloff, S.E. (eds.) Honeybees of Asia, pp. 227–255. Springer-Verlag Berlin, Heidelberg
Qiao, D., Seidler, F.J., Slotkin, T.A. (2005) Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharm. 206(1), 17–26
Ranjbar, A., Solhi, H., Mashayekhi, F.J., Susanabdi, A., Rezaie, A., Abdollahi, M. (2005) Oxidative stress in acute human poisoning with organophosphorus insecticides; a case control study. Environ. Toxicol. Pharmacol. 20(1), 88–91
Schreinemachers, P., Tipraqsa, P. (2012) Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 37(6), 616–626
Schriever, C.A., Callaghan, A., Biggs, J.P., Liess, M. (2008) Freshwater Biological Indicator of Pesticide Contamination. Environment Agency, Rio House
Smirle, M.J., Winston, M.L. (1988) Detoxifying enzyme activity in worker honeybees: an adaptation for foraging in contaminated ecosystems. Can. J. Zool. 66(9), 1938–1942
Smirle, M.J. (1988) Insecticide resistance mechanism in the honey bee, Apis mellifera L. Ph.D. Thesis, Graduate Faculty, Simon Fraser University, Vancouver.
Stanley, J., Chandrasekaran, S., Preetha, G., Kuttalam, S. (2010) Toxicity of diafenthiuron to honey bees in laboratory, semi-field and field conditions. Pest Manag. Sci. 66(5), 505–510
Starr, C.K., Schmidt, P.J., Schmidt, J.O. (1987) Nest-site preference of the giant honey bee, Apis dorsata (Hymenoptera : Apidae), in Borneo. Pan-Pac. Entomol. 63(1), 37–42
Stefanidou, M., Athanaselis, S., Koutselinis, A. (2003) The toxicology of honey bee poisoning. Vet. Hum. Toxicol. 45(5), 261–265
Visser, A., Blacquière, T. (2010) Survival rate of honeybee (Apis mellifera) workers after exposure to sublethal concentrations of imidacloprid. Proc Neth. Entomol. Soc. Meet. 21
Velki, M., Kodrík, D., Večeřa, J., Hackenberger, B.K., Socha, R. (2011) Oxidative stress elicited by insecticides: a role for the adipokinetic hormone. Gen. Comp. Endocrinol. 172(1), 77–84
Villa, S., Vighi, M., Finizio, A., Serini, G.B. (2000) Risk assessment for honeybees from pesticide-exposed pollen. Ecotoxicology 9, 287–297
Vorbach, C., Harrison, R., Capecchi, M.R. (2003) Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 24(9), 512–517
Wajner, M., Harkness, R.A. (1989) Distribution of xanthine dehydrogenase and oxidase activities in human and rabbit tissues. Biochim. Biophys. Acta 991(1), 79–84
Welinder, C., Ekblad, L. (2011) Coomassie staining as loading control in western blot analysis. J. Proteome Res. 10(3), 1416–1419
Whitehorn, P.R., O’Connor, S., Wackers, F.L., Goulson, D. (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336(6079), 351–352
Winfree, R., Aguilar, R., Vázquez, D.P., LeBuhn, G., Aizen, M.A. (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90(8), 2068–2076
Yu, S.J., Robinson, F.A., Nation, J.L. (1984) Detoxification capacity in the honey bee, Apis mellifera L. Pestic. Biochem. Physiol. 22, 360–368
Zhang, J.-W., Lv, G.-C., Zhao, Y. (2010) The significance of the measurement of serum xanthine oxidase and oxidation markers in patients with acute organophosphorus pesticide poisoning. J. Int. Med. Res. 38(2), 458–465
ACKNOWLEDGMENTS
The study was supported by an INSPIRE fellowship from the Department of Science & Technology, Govt. of India to Priyadarshini Chakrabarti; the Department for Environment, Food and Rural Affairs (DEFRA) Darwin Initiative grant (1662) to Parthiba Basu and Barbara Smith, and a research grant to Sagartirtha Sarkar from the Department of Science & Technology, Govt. of India. Soumik Chatterjee, Debaditya Kumar, Ritam Bhattacharya and Arnob Chatterjee helped in obtaining bee samples. Dr. Kaberi Samanta helped with the GIS maps. The Department of Agriculture, Governments of Odisha and West Bengal helped by providing the cropping intensity data. SGS India Private Limited analysed pesticide residues in soil and honey bee bodies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Stress oxydatif provoqué par des pesticides sur des populations indigènes d’abeilles, élevées en laboratoire et en champ, dans des zones d’agriculture intensive de deux Etats de l’est de l’Inde
Apis cerana / Apis dorsata / enzyme / dismutase / catalase / oxydase / insecticide
Durch Pestizide verursachter oxydativer Stress bei Labor. und Feldpopulationen einheimischer Honigbeienen entlang zweier intensiver Agrarlandschaften in zwei ostindischen Staaten
Apis cerana / Apis dorsata / Enzyme / Superoxid-Dismutase / Xanthin-Oxidase / Katalase / Insektizid
APPENDIX
APPENDIX
List of abbreviations used in methodology section:
Abbreviation | Full terminology | Abbreviation | Full terminology |
HIC | High intensity cropping | XOX | Xanthine oxidase |
LIC | Low intensity cropping | PBS | Phosphate buffered saline |
LCMS/MS | Liquid chromatography–mass spectrometry / mass spectrometry | NaCl | Sodium chloride |
GC | Gas chromatography | NP40 | Tergitol-type NP-40 (nonylphenoxypolyethoxylethanol) |
EPA3540C and EPA8081A procedures | Environmental Protection Agency methods 3540C and 8081A; followed by SGS India Pvt. Ltd. | EDTA | Ethylenediaminetetraacetic acid |
OP | Organophosphorus pesticide | SDS-PAGE | Sodium dodecyl sulfate—Polyacrylamide gel electrophoresis |
EC | Effective Concentration | PVDF | Polyvinylidenedifluoride |
SP | Synthetic pyrethroid | HRP | Horseradish peroxidase |
ES | Endosulfan pesticide | DTPA | Diethylene triamine pentaacetic acid |
Experimental treatments | D0 (control) D1 (5 % OP + 1.5 % SP + 5.5 % ES) D2 (12.5 % OP + 4 % SP + 15 % ES) D3 (20 % OP + 6.5 % SP + 23.5 % ES) D4 (25 % OP + 8 % SP + 29 % ES) D5 (30 % OP + 10 % SP + 35 % ES) | Tris–HCl | Tris (2-Amino-2-hydroxymethyl-propane-1,3-diol)—Hydrochloric acid |
SOD | Superoxide dismutase | CAT | Catalase |
Rights and permissions
About this article
Cite this article
Chakrabarti, P., Rana, S., Sarkar, S. et al. Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 46, 107–129 (2015). https://doi.org/10.1007/s13592-014-0308-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-014-0308-z