Abstract
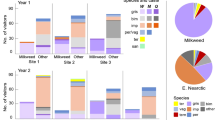
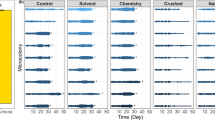
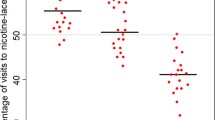
Flowers offer habitats for bacterial communities that are often characterized by low diversities but high densities. The composition of these communities and the dissemination of bacteria between flowers receive increasing attention, whereas the ecological functions of flower-associated but non-phytopathogenic bacteria remain understudied. We screened bacteria isolated from nectar, petals and leaves of two plant species for their potential to affect flower–visitor interactions. We took advantage of the proboscis extension reflex (PER) of bumblebees evoked by sugar and investigated whether bacteria associated with the reward may interrupt this reflex. Cultivated bacteria were transferred into a watery glucose solution in increasing densities and their effect on the proportion of bumblebees displaying the PER after antennal contact with glucose solutions and bacteria was scored. In all but one trial, the proportion of bumblebees that accepted the watery glucose solution was negatively correlated with the bacterial density. Nearly half of the bacteria tested evoked avoidance at naturally occurring densities. Our results suggest that bacteria colonizing flowers have the potential to negatively affect the reproduction of plants via reduced visits by pollinators.


Similar content being viewed by others
References
Adler, L.S. (2000) The ecological significance of toxic nectar. Oikos 91, 409–420
Alvarez-Perez, S., Herrera, C.M. (2013) Composition, richness and nonrandom assembly of culturable bacterial-microfungal communities in floral nectar of Mediterranean plants. FEMS Microbiol. Ecol. 83, 685–699
Alvarez-Perez, S., Herrera, C.M., de Vega, C. (2012) Zooming-in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol. Ecol. 80, 591–602
Anfora, G., Rigosi, E., Frasnelli, E., Ruga, V., Trona, F., Vallortigara, G. (2011) Lateralization in the invertebrate brain: left-right asymmetry of olfaction in bumble bee, Bombus terrestris. Plos One 6, e18903
Belisle, M., Peay, K.G., Fukami, T. (2012) Flowers as islands: spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird-pollinated shrub. Microb. Ecol. 63, 711–718
Brysch-Herzberg, M. (2004) Ecology of yeasts in plant–bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 50, 87–100
Buban, T., Orosz-Kovacs, Z., Farkas, A. (2003) The nectary as the primary site of infection by Erwinia amylovora (Burr.). Plant Syst. Evol. 238, 183–194
Carter, C., Thornburg, R.W. (2004) Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 9, 320–324
Crawley M. J. (2005) Statistics - An introduction using R. John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England.
Davis, T.S., Crippen, T.L., Hofstetter, R.W., Tomberlin, J.K. (2013) Microbial volatile emissions as insect semiochemicals. J. Chem. Ecol. 39, 840–859
Ercolani, G.L. (1991) Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb. Ecol. 21, 35–48
Evangelista, C., Kraft, P., Dacke, M., Reinhard, J., Srinivasan, M.V. (2010) The moment before touchdown: landing manoeuvres of the honeybee Apis mellifera. J. Exp. Biol. 213, 262–270
Ezenwa, V.O., Gerardo, N.M., Inouye, D.W., Medina, M., Xavier, J.B. (2012) Animal behavior and the microbiome. Science 338, 198–199
Fouks, B., Lattorff, H.M.G. (2011) Recognition and avoidance of contaminated flowers by foraging bumblebees (Bombus terrestris). Plos One 6, e26328
Fridman, S., Izhaki, I., Gerchman, Y., Halpern, M. (2012) Bacterial communities in floral nectar. Environ. Microbiol. Rep. 4, 97–104
Fuernkranz, M., Lukesch, B., Müller, H., Huss, H., Grube, M., Berg, G. (2012) Microbial diversity inside pumpkins: microhabitat-specific communities display a high antagonistic potential against phytopathogens. Microb. Ecol. 63, 418–428
Haupt, S.S. (2004) Antennal sucrose perception in the honey bee (Apis mellifera L.): behaviour and electrophysiology. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 190, 735–745
Herrera, C.M., Garcia, I.M., Perez, R. (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89, 2369–2376
Herrera, C.M., Pozo, M.I., Medrano, M. (2013) Yeasts in nectar of an early-blooming herb: sought by bumble bees, detrimental to plant fecundity. Ecology 94, 273–279
Huang, M., Sanchez-Moreiras, A.M., Abel, C., Sohrabi, R., Lee, S., Gershenzon, J., Tholl, D. (2012) The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-b-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 193, 997–1008
Jensen, G.B., Hansen, B.M., Eilenberg, J., Mahillon, J. (2003) The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5, 631–640
Johnson, K.B., Stockwell, V.O., Mclaughlin, R.J., Sugar, D., Loper, J.E., Roberts, R.G. (1993) Effect of Antagonistic Bacteria on Establishment of Honey Bee-Dispersed Erwinia-Amylovora in Pear Blossoms and on Fire Blight Control. Phytopathology 83, 995–1002
Junker, R.R., Tholl, D. (2013) Volatile organic compound mediated interactions at the plant-microbe interface. J. Chem. Ecol. 39, 810–825
Junker, R.R., Loewel, C., Gross, R., Dötterl, S., Keller, A., Blüthgen, N. (2011) Composition of epiphytic bacterial communities differs on petals and leaves. Plant Biol. 13, 918–924
Kai, M., Haustein, M., Molina, F., Petri, A., Scholz, B., Piechulla, B. (2009) Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81, 1001–1012
Knuth, P. (1908) Handbook of flower pollination. Clarendon, Oxford
Krimm, U., Abanda-Nkpwatt, D., Schwab, W., Schreiber, L. (2005) Epiphytic microorganisms on strawberry plants (Fragaria ananassa cv. Elsanta): identification of bacterial isolates and analysis of their interaction with leaf surfaces. FEMS Microbiol. Ecol. 53, 483–492
Lachance, M.A., Starmer, W.T., Rosa, C.A., Bowles, J.M., Barker, J.S.F., Janzen, D.H. (2001) Biogeography of the yeasts of ephemeral flowers and their insects. Fems Yeast Res. 1, 1–8
Leroy P. D., Sabri A., Heuskin S., Thonart P., Lognay G., Verheggen F. J., Francis F., Brostaux Y., Felton G. W., Haubruge E. (2011) Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat Commun 2, doi:10.1038/ncomms1347
Lindow, S.E., Brandl, M.T. (2003) Microbiology of the phyllosphere. Appl. Environ. Microb. 69, 1875–1883
Lunau, K., Unseld, K., Wolter, F. (2009) Visual detection of diminutive floral guides in the bumblebee Bombus terrestris and in the honeybee Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 195, 1121–1130
Maccagnani, B., Giacomello, F., Fanti, M., Gobbin, D., Maini, S., Angeli, G. (2009) Apis mellifera and Osmia cornuta as carriers for the secondary spread of Bacillus subtilis on apple flowers. Biocontrol 54, 123–133
Mattila, H.R., Rios, D., Walker-Sperling, V.E., Roeselers, G., Newton, I.L.G. (2012) Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. Plos One 7
Ondov B. D., Bergman N. H., Phillippy A. M. (2011) Interactive metagenomic visualization in a Web browser. Bmc Bioinforma. 12, doi:10.1186/1471-2105-1112-1385
Ponnusamy, L., Xu, N., Nojima, S., Wesson, D.M., Schal, C., Apperson, C.S. (2008) Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 105, 9262–9267
Pozo, M.I., Lachance, M.A., Herrera, C.M. (2012) Nectar yeasts of two southern Spanish plants: the roles of immigration and physiological traits in community assembly. FEMS Microbiol. Ecol. 80, 281–293
R Development Core Team (2011) R: A language and environment for statistical computing. ed.^eds.), p.^pp. R Foundation for Statistical Computing, Vienna.
Sanchez, M.G.D. (2011) Taste Perception in Honey Bees. Chem. Senses 36, 675–692
Schulz, S., Dickschat, J. (2007) Bacterial volatiles: the smell of small organisms. Nat. Prod. Rep. 24, 814–842
Shade, A., McManus, P.S., Handelsman, J. (2013) Unexpected diversity during community succession in the apple flower microbiome. mBio 4, e00602–e00612
Stensmyr, M.C., Dweck, H.K.M., Farhan, A., Ibba, I., Strutz, A., Mukunda, L., Linz, J., Grabe, V., Steck, K., Lavista-Llanos, S., Wicher, D., Sachse, S., Knaden, M., Becher, P.G., Seki, Y., Hansson, B.S. (2012) A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357
van der Steen, J.J.M., Langerak, C.J., van Tongeren, C.A.M., Dik, A.J. (2004) Aspects of the use of honeybees and bumblebees as vector of antagonistic micro-organisms in plant disease control. Proc. Neth. Entomol. Soc. 15, 41–46
Vannette, R.L., Gauthier, M.-P.L., Fukami, T. (2012) Nectar bacteria, but not yeast, weaken a plant–pollinator mutualism. Proc. R. Soc. B 280, 20122601
Wang, Q., Garrity, G.M., Tiedje, J.M., Cole, J.R. (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267
Yang, C.-H., Crowley, D.E., Borneman, J., Keen, N.T. (2001) Microbial phyllosphere populations are more complex than previously realized. Proc. Natl. Acad. Sci. U. S. A. 98, 3889–3894
Acknowledgments
We thank Christina Loewel for helpful advices and Karl Köhrer for technical support. The project was supported by the Deutsche Forschungsgemeinschaft (DFG, JU 2856/1-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Bernd Grünewald
Réponses négatives densité-dépendantes des bourdons aux bactéries isolées à partir des fleurs
Aversion / bactérie / Bombus terrestris / interactions plante–bactérie–animal / PER / réflexe d’extension du proboscis
Hummeln vermeiden Bakterien auf Blüten in natürlichen Dichten
Abneigung / Bacilli / Bombus terrestris / Pflanzen–Bakterien–Tier Interaktionen / Rüsselreflex
Rights and permissions
About this article
Cite this article
Junker, R.R., Romeike, T., Keller, A. et al. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 45, 467–477 (2014). https://doi.org/10.1007/s13592-013-0262-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-013-0262-1