Abstract
There is concern about the potential for transgene contamination in wild type (WT) plants via pollen-mediated gene flow from transgenic plants. In this study, we investigate pollen-mediated gene flow using tobacco transgenic lines carrying recombinant protein GA733-FcK, which is a putative candidate for producing colorectal cancer vaccine. Transgenic and WT plants were grown in greenhouse, with WT plants placed 0.3, 1, 5, 10, and 20 m away from transgenic plants. Seeds were harvested from randomly selected WT plants and sown on germination media supplemented with or without kanamycin. At 30 days after sowing, none of the WT seedlings produced true leaves and roots when grown on selective media. Polymerase chain reaction analysis revealed that GA733-FcK and nptII genes were expressed in shoots of transgenic plants but not of WT plants. Expression of GA733-FcK and nptII proteins was also abolished in WT leaves when compared to that of transgenic plants. Our findings suggested that there is low risk of pollen-mediated gene flow from transgenic plants expressing GA733-FcK when grown in greenhouse conditions.
Similar content being viewed by others
1 Introduction
Gene flow refers to the transfer of genes or genetic material from one plant to another (Ellstrand 2003; Chandler and Dunwell 2008; Heuberger et al. 2011; Chen et al. 2016) and occurs via three routes; pollen, seed, and vegetative propagule-mediated (Qaim 2009; Kang et al. 2016). Pollen-mediated gene flow is influenced by physical and environmental conditions such as the distance between pollen donors and recipients, temperature, relative humidity and strength and direction of air flow (Husken et al. 2010). Gene flow is a major factor affecting the purity of crop cultivars (Dong et al. 2016) and therefore is assessed when conducting risk assessments of genetically modified (GM) plants (Chandler and Dunwell 2008; Jamal et al. 2012; Bohn et al. 2016).
In recent years, the increased use of GM plants has required the establishment of biosafety rules to ensure the low escape of exogenous genes from GM to non-GM crops (Kwit et al. 2011; DiFazio et al. 2012). Several previous studies have investigated pollen-mediated gene flow in the field (Goggi et al. 2007; Baltazar et al. 2015; Miroshnichenko et al. 2016); however, no data are available on the contamination risk via pollen under greenhouse conditions. When GM plants are cultivated in restricted spaces, such as a greenhouse, the risk of pollen contamination is lower than in external environment because temperature, wind and humidity are adjusted to restrict pollen dispersal. Yet, pollen contamination, in greenhouse conditions, may occur due to the narrow spacing between plants.
GM technology enables the production of compounds and molecules with beneficial properties in high amounts. Previously, we reported the potential of using plants to produce the vaccine candidate GA733, which is an epithelial cell adhesion molecule highly expressed in colorectal cancer cells (Mosolits et al. 1999). The recombinant protein GA733-FcK produced in plants was able to induce immunogenic responses in animal models (Lu et al. 2012; Lim et al. 2015). In this study, we investigated whether growth of tobacco transgenic plants expressing the GA733-FcK under greenhouse conditions resulted in contamination of wild type (WT) plants via pollen-mediated gene flow.
2 Materials and methods
2.1 Plant materials and experimental fields
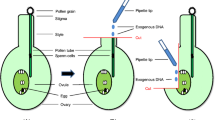
WT and transgenic Nicotiana tabacum plants expressing the epidermal cell adhesion molecule GA733, which is a cancer vaccine candidate, were used (Kim et al. 2009; Lu et al. 2012). To monitor the flow of transgenes from transgenic to WT plants, both screening and selection were undertaken using GA733 fused to an IgG HC fragment (GA733-Fc) as target gene and the neomycin phosphotransferase II (nptII) gene as a selective marker (Lu et al. 2012). Tobacco plants were grown in greenhouse in 12 h light/12 h dark photoperiod. WT plants were placed around transgenic plants at distance intervals of 0.3, 1, 5, 10, and 20 m. Pollen-mediated gene flow from transgenic to WT plants assessed from plants having similar growth patterns and flowering times.
2.2 In vitro seed germination
Seeds were randomly collected from WT plants and were surface sterilized with in 20% ethanol and 10% sodium hypochlorite. Seeds were planted on Murashige and Skoog (MS) medium supplemented with 30 g L−1 sucrose, 6 g L−1 phyto agar, and 4.8 g L−1 MS B5 vitamin (Duchefa Biochemie, Haarlem, Netherlands), and with or without 100 mg L−1 kanamycin. Germinated seedlings were observed 4 weeks after seed sowing, and leaves were harvested for polymerase chain reaction (PCR) and immunoblot analyses.
2.3 Genomic DNA isolation and PCR analysis
The presence of transgenes in WT plant seeds was monitored using PCR. Genomic DNA was extracted from the leaves (100 mg) of 30-day-old transgenic and WT plant seedlings using DNA extraction kit (RBC Bioscience, Seoul, Korea) according to the manufacturer’s recommendations. Primer pairs used to amplify the GA733-FcK gene were 5′-CATGCCATGGATGGCTACTCAACG-3′ and 5′-ATCACAGAGCA TGAGAAGACGTTC-3′. Primer pairs used to amplify the nptII gene were 5′-CTCCCA ATCAGGCTTGATCCCCAG-3′ and 5′-CCTGCTAAGGTATATAAGCTGG TG-3′. The EF1α gene (Actin) was used as a reference gene. PCR analysis was conducted more than three times.
2.4 Immunoblot analysis
Total soluble protein was extracted from 100 mg of fresh leaves from transgenic and WT seedlings by cryomilling. The homogenized plant samples were mixed with 300 μL of 1X PBS (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, and 2 mM KH2PO4). Leaf extracts were boiled with 5 × protein loading buffer (1 M Tris-HCl, 50% glycerol, 10% SDS, 5% 2-mercaptoethanol, and 0.1% bromophenol blue) for 10 min and then cooled for 2 min. Total soluble proteins (16 μL) were mixed with 4 μL loading buffer and were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10% SDS) before transferred to nitrocellulose membrane (Millipore, Billerica, MA). The nitrocellulose membranes were blocked in 5% skimmed milk (Sigma, St. Louis, MO) prepared in 1X phosphate-buffered saline (PBS) for 2 h at room temperature. Proteins were detected with mouse anti-EpCAM and rabbit anti-nptII IgGs, which recognize the GA733 and nptII proteins, respectively. Blots were incubated with either horseradish peroxidase-linked goat anti-mouse IgG Fc or anti-rabbit IgG Fc antibodies (Abcam Inc., Cambridge, MA) at 1:5000 dilution in 1X PBS for 2 h. Immunoblot were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and visualized by exposing the membrane to X-ray film (Fuji, Tokyo, Japan). Leaf tissue extracted from non-transgenic plants was used as a negative control.
3 Results
To investigate whether transgene flow occurs under greenhouse conditions, WT plants were grown on soil pots and placed at 0.3, 1, 5, 10, and 20 m away from GA733-FcK expressing plants. Transgenic and WT plants were selected to be at similar developmental stages and flowering periods. All plants started flowering at 14 weeks after planting and retained their blossoms until 30 days after onset of flowering (Fig. 1b). Figure 2 presents the experimental design with transgenic plants being located at the center of the greenhouse and WT plants being spaced at different intervals with the longest being 20 m away (Fig. 2). During the flowering period, the average temperature during the day and night was 30 °C and 15 °C, respectively (Fig. 3). The average relative humidity was 60% at daytime while it dropped to 28% at nighttime (Fig. 3). To confirm whether GA733-FcK transgene flow occurred between the transgenic and WT plants, seeds were randomly collected from WT plants located at 0.3, 1, 5, 10, and 20 m distance intervals and were germinated on either kanamycin-supplemented or control media (Fig. 4). In spite of separation distances, all WT plant seeds produced four true leaves and healthy roots when grown on media without kanamycin, whereas growth of WT plants on media supplemented with kanamycin impaired the production of four true leaves (Fig. 4). By contrast, growth of transgenic plant seeds in control and selective media did not affect the production of four true leaves and roots (Fig. 4). Both GA733-FcK and nptII genes were expressed in transgenic plants (Fig. 5, top), whereas no gene expression was detected in WT plants (Fig. 5, middle). Actin transcripts were detected at similar levels in both transgenic and WT plants (Fig. 5, bottom). Immunoblot analysis of total soluble extracts confirmed the expression of GA733-FcK (~ 68 kDa) and nptII (~ 30 kDa) proteins in transgenic plants while no proteins were present in the WT plants (Fig. 6a, b).
Experimental design used to assess pollen-mediated gene flow between wild-type (WT) and transgenic tobacco plants. a Schematic diagram indicates the distance intervals of 0.3, 1, 5, 10, and 20 m between WT and transgenic plants. b Representative images of transgenic and WT tobacco plants grown under greenhouse conditions
Positioning pattern of transgenic and wild type (WT) plants grown under greenhouse conditions. a Schematic diagram of the greenhouse showing the positions 0.3, 1, 5, 10, and 20 m, where WT plants were positioned relative to transgenic plants. b Representative images of flowering WT plants located at 0.3, 1, and 5 m. c Representative images of flowering WT plants located at positions 0.3, 1, 5, 10, and 20 m
Phenotype comparison of growth and germination of seedlings from transgenic and WT plants. In vitro seed culture of transgenic and wild type (WT) plants grown at 0.3, 1, 5, 10, and 20 m distance intervals; images show 30-day old seedlings grown on media supplemented with (+) or without (−) kanamycin
Identification of GA733-FcK and nptII proteins in transgenic and WT plants. Immunodetection of GA733-FcK (68 kDa) (a) and nptII (30 kDa) (b) carried out using mouse anti-EpCAM and rabbit anti-nptII IgGs, respectively. c Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of leaf extracts from transgenic (T) and WT plants grown at 0.3, 1, 5, 10, and 20 m distance intervals
4 Discussion
Here we demonstrated that pollen-mediated gene flow did not occur between transgenic plants expressing the recombinant GA733-FcK protein and WT plants when grown under greenhouse conditions. GA733-FcK has been considered to be a good candidate for colorectal cancer vaccination and has been successfully expressed in transgenic plants (Lu et al. 2012; Lim et al. 2015) and insect cell systems (Oh et al. 2011; Kim et al. 2015; Lee et al. 2016). Gene flow from transgenic to WT plants has often been considered to be a potential risk in the biotechnological applications (Snow 2002; Song et al. 2015). Thus, it is essential to investigate whether gene flow of transgenes occurs from transgenic to WT plants.
The current study was conducted using transgenic and WT tobacco plants grown in greenhouse environment. Growth during the late summer and early autumn was chosen to ensure the synchronous production of flowers from both transgenic and WT plants. During the experimental period, temperatures sometimes reached high values and humidity levels varied from 35% to 85%; despite these conditions, plants did not exhibit any physiological problems. In the present study, we did not observe any gene flow from transgenic to WT plants, at any of the separation distances (0.3, 1, 5, 10, and 20 m between WT and transgenic plants) under greenhouse conditions. It was particularly important to confirm that there was no gene flow from transgenic to WT plants at a distance of 30 cm apart. These results are not unexpected given the ability of tobacco plants to self-pollinate and the fact that greenhouse conditions restrict pollination by wind transport (Serrat et al. 2013). Tobacco pollen can be transferred between flowers by bees and other insects (Loureiro et al. 2016). However, in the present study, we did not detect any flow of transgenes. We speculate that insects were well controlled in the greenhouse, therefore preventing any transfer by insects of pollen from transgenic to WT plants. Overall, our current data have implications for growing transgenic tobacco plants in greenhouse conditions: that is, no gene flow occurs between transgenic and WT plants, if grown under the containment conditions outlines in this paper, with good control of insects.
References
Baltazar BM, Espinoza LC, Banda AE, Martinez JMD, Tiznado JAG, Garcia JG, Gutierrez MA, Rodriguez JLG, Diaz OH et al (2015) Pollen-mediated gene flow in maize: implications for isolation requirements and coexistence in Mexico, the center of origin of maize. PLoS ONE 10:e0131549
Bohn T, Aheto DW, Mwangala FS, Fischer K, Bones IL, Simoloka C, Mbeule I, Schmidt G, Breckling B (2016) Pollen-mediated gene flow and seed exchange in small-scale Zambian maize farming, implications for biosafety assessment. Sci Rep 6:34483
Chandler S, Dunwell JM (2008) Gene flow, risk assessment and the environmental release of transgenic plants. Crit Rev Plant Sci 27:25–49
Chen J, Ying GG, Wei XD, Liu YS, Liu SS, Hu LX, He LY, Chen ZF, Chen FR et al (2016) Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: effect of flow configuration and plant species. Sci Total Environ 571:974–982
DiFazio SP, Leonardi S, Slavov GT, Garman SL, Adams WT, Strauss SH (2012) Gene flow and simulation of transgene dispersal from hybrid poplar plantations. New Phytol 193:903–915
Dong S, Liu Y, Yu C, Zhang Z, Chen M, Wang C (2016) Investigating pollen and gene flow of WYMV-resistant transgenic wheat N12-1 using a dwarf male-sterile line as the pollen receptor. PLoS ONE 11:e0151373
Ellstrand NC (2003) Current knowledge of gene flow in plants: implications for transgene flow. Philos Trans R Soc Lond B Biol Sci 358:1163–1170
Goggi AS, Lopez-Sanchez H, Caragea P, Westgate M, Arritt R, Clark CA (2007) Gene flow in maize fields with different local pollen densities. Int J Biometeorol 51:493–503
Heuberger S, Crowder DW, Brevault T, Tabashnik BE, Carriere Y (2011) Modeling the effects of plant-to-plant gene flow, larval behavior, and refuge size on pest resistance to Bt cotton. Environ Entomol 40:484–495
Husken A, Prescher S, Schiemann J (2010) Evaluating biological containment strategies for pollen-mediated gene flow. Environ Biosafety Res 9:67–73
Jamal A, Lee J-H, Lee K-J, Oh D-B, Kim D-S, Lee K-K, Choo Y-K, Hwang K-A, Ko K (2012) Chimerism of multiple monoclonal antibodies expressed in a single plant. Hortic Environ Biotechnol 53:544–551
Kang Y, Shin YK, Park S-W, Ko K (2016) Effect of nitrogen deficiency on recombinant protein production and dimerization and growth in transgenic plants. Hortic Environ Biotechnol 57:299–307
Kim KI, Yoo KH, Chung HY, Lee JH, Lee HH, Seok YJ, Hwang-Bo J, Cui EJ, Ko KS et al (2009) Expression of recombinant colorectal cancer antigen GA733-2 in plants and its immune response in mice. J Biosci Bioeng 108:S14–S15
Kim DS, Qiao L, Lee KJ, Ko K (2015) Optimization of colorectal cancer vaccine candidate protein GA733-Fc expression in a baculovirus-insect cell system. Entomol Res 45:39–48
Kwit C, Moon HS, Warwick SI, Stewart CN (2011) Transgene introgression in crop relatives: molecular evidence and mitigation strategies. Trends Biotechnol 29:284–293
Lee JH, Qiao L, Song MR, Ko K (2016) Murine response studies of insect cell (Sf9) expressed recombinant colorectal cancer vaccine candidate using surface plasmon resonance studies. Entomol Res 46:5–14
Lim CY, Lee KJ, Oh DB, Ko K (2015) Effect of the developmental stage and tissue position on the expression and glycosylation of recombinant glycoprotein GA733-FcK in transgenic plants. Front Plant Sci 5:778
Loureiro I, Garcia-Ruiz E, Gutierrez E, Gomez P, Escorial MC, Chueca MC (2016) Pollen-mediated gene flow in the cultivation of transgenic cotton under experimental field conditions in Spain. J Indcrop 85:22–28
Lu Z, Lee KJ, Shao Y, Lee JH, So Y, Choo YK, Oh DB, Hwang KA, Oh SH et al (2012) Expression of GA733-Fc fusion protein as a vaccine candidate for colorectal cancer in transgenic plants. J Biomed Biotechnol 2012:11
Miroshnichenko D, Pushin A, Dolgov S (2016) Assessment of the pollen-mediated transgene flow from the plants of herbicide resistant wheat to conventional wheat (Triticum aestivum L.). Euphytica 209:71–84
Mosolits S, Harmenberg U, Rudén U, Ohman L, Nilsson B, Wahren B, Fagerberg J, Mellstedt H (1999) Autoantibodies against the tumour-associated antigen GA733-2 in patients with colorectal carcinoma. Cancer Immunol Immunother 47:315–320
Oh E-Y, Kim YK, Park D-Y, Lu Z, Choo YK, Han YS, Park JM, Ko K (2011) Expression of recombinant proteins in plants by using baculovirus vectors. Hortic Environ Biotechnol 52:95–104
Qaim M (2009) The economics of genetically modified crops. Ann Rev Resour Econ 1:665–693
Serrat X, Esteban R, Penas G, Catala MM, Mele E, Messeguer J (2013) Direct and reverse pollen-mediated gene flow between GM rice and red rice weed. Aob Plants. https://doi.org/10.1093/aobpla/plt050
Snow AA (2002) Transgenic crops—why gene flow matters. Nat Biotechnol 20:542
Song I, Kim DS, Kim MK, Jamal A, Hwang K-A, Ko K (2015) Comparison of total soluble protein in various horticultural crops and evaluation of its quantification methods. Hortic Environ Biotechnol 56:123–129
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1A6A3A11930180), National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2017R1A2A2A0569788), Korean Rural Development Administration (Code#PJ0134372018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, DS., Song, I. & Ko, K. Low risk of pollen-mediated gene flow in transgenic plants under greenhouse conditions. Hortic. Environ. Biotechnol. 59, 723–728 (2018). https://doi.org/10.1007/s13580-018-0074-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0074-3