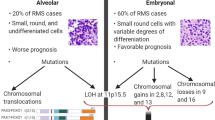
Abstract
Receptor tyrosine kinases (RTKs) serve as molecular targets for the development of novel personalized therapies in many malignancies. In the present study, expression pattern of receptor tyrosine kinases and its clinical significance in orbital RMS has been explored. Eighteen patients with histopathologically confirmed orbital RMS formed part of this study. Comprehensive q-PCR gene expression profiles of 19 RTKs were generated in the cases and controls. The patients were followed up for 59.53 ± 20.93 years. Clustering and statistical analysis tools were applied to identify the significant combination of RTKs associated with orbital rhabdomyosarcoma patients. mRNA overexpression of RTKs which included MET, AXL, EGFR was seen in 60–80% of cases; EGFR3, IGFR2, FGFR1, RET, PDGFR1, VEGFR2, PDGFR2 in 30–60% of cases; and EGFR4, FGFR3,VEGFR3 and ROS,IGFR1, EGFR1, FGFR2, VEGFR1 in 10–30% of cases. Immunoexpression of MET was seen in 89% of cases. A significant association was seen between MET mRNA and its protein expression. In all the cases MET gene expression was associated with worst overall survival (P = 0.03).There was a significant correlation of MET mRNA expression with RET, ROS, AXL, FGFR1, FGFR3, PDGFR1, IGFR1, VEGFR2, and EGFR3 genes. Association between MET gene and collective expression of RTKs was further evaluated by semi-supervised gene cluster analysis and Principal component analysis, which showed well-separated tumor clusters. MET gene overexpression could be a useful biomarker for identifying high risk orbital rhabdomyosarcoma patients. Well-separated tumor clusters confirmed the association between MET gene and collective expression of RTK genes. Therefore, the therapeutic potential of multi-kinase inhibitors targeting MET and the 9 other significant RTKs needs to be explored.




Similar content being viewed by others
References
Bejar DE, Huh WW. Rhabdomyosarcoma in adolescent and young adult patients:currentperspectives. Health Med Ther. 2014;5:115–25.
Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003;48(1):39–57.
Terezakis SA, Wharam MD. Radiotherapy for rhabdomyosarcoma:indications and outcome. Clin Oncol R CollRadiol. 2013;25:27–35.
Viswanathan S, George S, Ramadwar M, et al. Extraconal orbital tumors in children—a spectrum. Virchows Arch. 2009;454:703–13.
Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–3.
Sohaib SA, Moseley I, Wright JE. Orbital rhabdomyosarcoma-the radiological characteristics. Clin Radiol. 1998;53:357–62.
Maurer HM, Beltangady M, Gehan EA, et al. The intergroup rhabdomyosarcoma study-IA final report. Cancer. 1988;61:209–20.
Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–34.
Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–57.
Zhenfang Du, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:58.
Krug M, Hilgeroth A. Recent advances in the development of multi- kinase inhibitors. Mini Rev Med Chem. 2008;8(13):1312–27.
Broekman F, Giovannetti E, Peters G. Tyrosine kinase inhibitors: multi- targeted or single-targeted? World J Clin Oncol. 2011;2(2):80–93.
Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non- small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol. 2020;38(5):488–95.
Janne PA, Neal JW, Camidge DR, et al. Antitumor activity of TAK-788 in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2019;37(15_suppl):9007.
Le X, Goldman JW, Clarke JM, et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol. 2020;38(15_suppl):9514.
Chia P, Mitchell P, Dobrovic A, et al. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. ClinEpidemiol. 2014;6:423–32.
Shaw A, Ou S, Bang Y, et al. Crizotinib in ROS1-rearranged non-small- cell lung cancer. N Engl J Med. 2014;371(21):1963–71.
Stirrups R. Neratinib and capecitabine for breast cancer brain metasta- ses. Lancet Oncol. 2019;20(4): e197.
Nasrazadani A, Brufsky A. Neratinib: the emergence of a new player in the management of HER2+ breast cancer brain metastasis. Future Oncol. 2020;16(7):247–54.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocel- lular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
Abou-Alfa G, Meyer T, Cheng A, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63.
Li Q, Qin S, Gu S, et al. Apatinib as second-line therapy in Chinese patients with advanced hepatocellular carcinoma: a randomized, placebo-controlled, double-blind, phase III study. J Clin Oncol. 2020;38(15):4507.
Choueiri T, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a ran- domised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–75.
Liu Y, Hu X, Jiang J, et al. A prospective study of apatinib in patients with extensive-stage small cell lung cancer after failure of two or more lines of chemotherapy. Oncologist. 2020;25(5):e833–42.
Poddubskaya E, Baranova M, Allina D, et al. Personalized prescription of tyrosine kinase inhibitors in unresectable metastatic cholangiocar- cinoma. Exp Hematol Oncol. 2018;7:21.
Gainor JFCG, Kim D-W, et al. Registrational dataset from the phase I/ II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15_suppl):9515.
Subbiah V, Hu MIN, Gainor JF, et al. Clinical activity of the RET inhibi- tor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors. J Clin Oncol. 2020;38(15_suppl):109.
Drilon A, Clark J, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47–51.
Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previ- ously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov. 2018;8(7):812.
AbbaspourBabaei M, Kamalidehghan B, Saleem M, et al. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des DevelTher. 2016;10:2443–59.
Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melano- mas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J ClinOncol. 2013;31(26):3182–90.
Mei L, Du W, Idowu M, et al. Advances and challenges on management of gastrointestinal stromal tumors. Front Oncol. 2018;8:135.
Lawrence WJ, Anderson JR, Gehan EA, et al. Pretreatment TNM staging of childhood rhabdomyosarcoma: a report of the intergroup rhabdomyosarcoma study group. Cancer. 1997;80(6):1165–70.
Hou J, Dong J, Sun L, et al. Inhibition of phosphorylated c-Met in rhabdomyosarcoma cell lines by a small molecule inhibitor SU11274. J Transl Med. 2011;9:64.
Lim L, Wu CC, Hsu YT, et al. Clinical significance of c-Met and phospho-c-Met (Tyr1234/1235) in ovarian cancer. Taiwan J Obstet Gynecol. 2019;2019(58):105e110.
Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA- 665752. Proc Natl Acad Sci USA. 2006;103:2316–21.
Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–8.
Anastasi S, Giordano S, Sthandier O, et al. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive met kinase activation on myogenic differentiation. J Cell Biol. 1997;137(5):1057–68.
Camassei FD, McDowell HP, Deloris MA, et al. Clinical significance of CXC chemokine receptor-4 and c-Met in childhood rhabdomyosarcoma. Clin Cancer Res. 2008;14(13):4119–27.
Paccez JD, Vogelsang M, Parker MI, et al. The receptor tyrosine kinase Axl in cancer: Biological functions and therapeutic implications. Int J Cancer. 2014;134:1024–33.
Huang F, Hurlburt W, Greer A, et al. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS- 754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70:7221–31.
Nicholson RI, Gee JMW, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–15.
Selvaggi G, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32.
Grass B, Wachtel M, Behnke S, et al. Immunohistochemical detection of EGFR, fibrillin-2, P-cadherin and AP2β as biomarkers for rhabdomyosarcoma diagnostics. Histopathology. 2009;54(7):873–9.
Ganti R, Skapek SX, Zhang J, et al. Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonalrhabdomyosarcoma. Mod Pathol. 2006;19(9):1213–20.
Wachtel M, Runge T, Leuschner I, et al. Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry. J Clin Oncol. 2006;24(5):816–22.
Ricci C, Landuzzi L, Rossi I, et al. Expression of HER/erbB family of receptor tyrosine kinases and induction of differen- tiation by glial growth factor 2 in human rhabdomyosarcoma cells. Int J Cancer. 2000;87(1):29–36.
Giovanni CD, Landuzzi L, Frabetti F, et al. Antisense epidermal growth factor receptor transfection impairs the proliferative ability of human rhabdomyosarcoma cells. Can Res. 1996;56(17):3898–901.
Danz YZ, Zhang Y, Li JP, et al. High VEGFR1/2 expression levels are predictors of poor survival in patients with cervical cancer. Medicine. 2017;96(1):e5772.
Juttner S, Wissmann C, Jons T, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J ClinOncol. 2006;24:228–40.
Arinaga M, Noguchi T, Takeno S, et al. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457–64.
Van Trappen PO, Steele D, Lowe DG, et al. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201:544–54.
Witte D, Thomas A, Ali N, et al. Expression of the vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal adenocarcinoma. Anticancer Res. 2002;22:1463–6.
Kurmasheva RT, Dudkin L, Billups C, et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Can Res. 2009;69(19):7662–71.
Maris M, Courtright J, Houghtonetal PJ. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(3):581–7.
Vilmar A, Santoni-Rugiu E, Garcia-Foncillas J, et al. Insulin-like growth factor receptor 1 mRNA expression as a prognostic marker in advanced non-small cell lung cancer. Anticancer Res. 2014;34:2991–6.
Thariat J, Bensadoun RJ, Etienne-Grimaldi MC, et al. Contrasted outcomes to gefitinib on tumoral IGF1R expression in head and neck cancer patients receiving postoperative chemoradiation (GORTEC trial 2004–02). Clin Cancer Res. 2012;18:5123–33.
Singh SK, Tan QW, Brito C, et al. Insulin-like growth factors I and II receptors in the breast cancer survival disparity among African-American women. Growth Horm IGF Res. 2010;20:245–54.
Makawita S, Ho M, Durbin AD, et al. Expression of insulin-like growth factor pathway proteins in rhabdomyosarcoma: IGF-2 expression is associated with translocation-negative tumors. Pediatr Dev Pathol. 2009;12(2):127–35.
Petricoin EF, Espina V, Araujo RP, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Can Res. 2007;67(7):3431–40.
Ayalon D, Glaser T, Werner H. Transcriptional regulation of IGF-I receptor gene expression by the PAX3- FKHR oncoprotein. Growth Hormon IGF Res. 2001;11(5):289–97.
Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic broblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–71.
Theillet C, Adelaide J, Louason G, et al. FGFRI and PLAT genes and DNA amplication at 8p12 in breast and ovarian cancers. Genes Chromosom Cancer. 1993;7:219–26.
Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62–93.
Byron SA, Gartside M, Powell MA, et al. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PLoS ONE. 2012;7: e30801.
Antoniou AC, Spurdle AB, Sinilnikova OM, et al. Common breast cancer-predisposition alleles are associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Am J Hum Genet. 2008;82:937–48.
Jang JH, Shin KH, Park JG. Mutations in broblast growth factor receptor 2 and broblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3.
Van Rhijn BW, Montironi R, Zwartho EC, et al. Frequent FGFR3 mutations in urothelial papilloma. J Pathol. 2002;198:245–51.
Chesi M, Nardini E, Brents LA, et al. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of broblast growth factor receptor 3. Nat Genet. 1997;16:260–4.
Goldstein M, Meller I, Orr-Urtreger A. FGFR1 over-expression in primary rhabdomyosarcoma tumors is associated with hypomethylation of a 5′ CpG island and abnormal expression of the AKT1, NOG, and BMP4 genes. Genes Chromosom Cancer. 2007;46(11):1028–38.
Hirotsu M, Setoguchi T, Matsunoshita Y, et al. Tumour formation by single fibroblast growth factor receptor 3- positive rhabdomyosarcoma-initiating cells. Br J Cancer. 2009;101(12):2030–7.
Taylor JG, Cheuk AT, Tsang PS, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Investig. 2009;119(11):3395–407.
Oseini AM, Roberts LR. PDGFR alpha: a new therapeutic target in the treatment of hepatocellular carcinoma? Expert OpinTher Targets. 2009;13:443.
Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247.
Ozawa T, Brennan CW, Wang L, et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24:2205.
Jones AV, Cross NC. Oncogenic derivatives of platelet-derived growth factor receptors. Cell Mol Life Sci. 2004;61:2912.
Fujino S, Miyoshi N, Ohue M, et al. Platelet-derived growth factor receptor-β gene expression relates to recurrence in colorectal cancer. Oncol Rep. 2018;39:2178–84.
Armistead PM, Salganick J, Roh JS, et al. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma: correlation with overall survival in 105 patients. Cancer. 2007;110(10):2293–303.
Blandford MC, Barr FG, Lynch JC, et al. Rhabdomyosarcomas utilize devel- opmental, myogenic growth factors for disease advantage: a report from the children’s oncology group. Pediatr Blood Cancer. 2006;46(3):329–38.
Taniguchi E, Nishijo K, McCleish AT, et al. PDGFR- a is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene. 2008;27(51):6550–60.
Chugh R, Wathen JK, Maki RG, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. 2009;27(19):3148–53.
Griseri P, Garrone O, Sardo AL, et al. Genetic and epigenetic factors affect RET gene expression in breast cancer cell lines and influence survival in patients. Oncotarget. 2016;7:26465–79.
Zeng Q, Cheng Y, Zhu Q, et al. The Relationship between over-expression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36:656–64.
Ban K, Feng S, Shao L, et al. RET signaling in prostate cancer. Clin Cancer Res. 2017;23:4885–96.
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010. https://doi.org/10.3109/10715761003667554.
Paik PK, Veillon R, Cortot AB, et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations. J Clin Oncol. 2019;37(15_suppl):9005.
Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383:931–43.
Wang Q, Yang S, Wang K, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;12(1):63.
Funding
This study was financially supported by research grant from University Grants Commission No. F.15-1/2017/PDFWM-2017-18-MAD-46619(SA-II).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
This study was conducted after approval from the Institute Ethics Committee, AIIMS, New Delhi Ref No IEC-45/02.02.2018,RP-02/2018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2023_993_MOESM1_ESM.tif
Supplementary file 1: Supplementary Figure1. Kaplan–Meier survival curves to show overall survival in rhabdomyosarcoma cases based on the RTKs gene expression. (TIF 112523 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chauhan, S., Sen, S., Irshad, K. et al. Receptor tyrosine kinase gene expression profiling of orbital rhabdomyosarcoma unveils MET as a potential biomarker and therapeutic target. Human Cell 37, 297–309 (2024). https://doi.org/10.1007/s13577-023-00993-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00993-5