Abstract
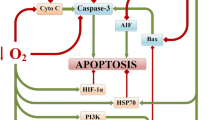
Intake of central nervous system (CNS) stimulants causes hypoxia and brain edema, which results in nerve cell death. However, no study has yet investigated the direct and continuous effects on nerve cells of CNS stimulants under hypoxia. Thus, based on autopsy cases, the effects of CNS stimulant drugs on the CNS were examined. The pathological changes in cultured nerve cells when various CNS stimulants were added under a hypoxic condition were also investigated. Five groups (Group A, stimulants; Group B, stimulants with psychiatric drugs; Group C, caffeine; Group D, psychiatric drugs; and Group E, no drugs) according to the detected drugs in autopsy cases were compared, and brain edema was evaluated using morphological findings. Furthermore, the number of dead cultured nerve cells was counted after the addition of drugs (4-aminopyridine (4-AP), caffeine, and ephedrine) under hypoxia (3% O2). Staining with anti-receptor-interacting protein 3 (RIP3) and other associated stains was also performed to investigate the neuronal changes in the brain. Group A showed significantly more brain edema than the other groups. In the culture experiments, the ratio of nerve cell death after the addition of 4-AP was the highest in the hypoxic condition. Groups with stimulants detected were stained more strongly by RIP3 immunostaining than by other staining. Addition of stimulants to cultured nerve cells in a persistent hypoxic condition led to severe cytotoxicity and nerve cell death. These findings suggest that necroptosis is involved in nerve cell death due to the addition of CNS stimulants in the hypoxic condition.









Similar content being viewed by others
References
Lutsch DJ, Camic CL, Jagim AR, et al. Effects of a multi-ingredient preworkout supplement versus caffeine on energy expenditure and feelings of fatigue during low-intensity treadmill exercise in college-aged males. Sports. 2020;8:E132. https://doi.org/10.3390/sports8100132.
United Nations Office on Drugs and Crime, World drug report 2020 (United Nations publication, Sales No. E.20.XI.6). Austria: UNODC Publications; 2020. https://wdr.unodc.org/wdr2020/index2020.html. Accessed 25 Mar 2022.
Sanctis V, Soliman N, Soliman AT, et al. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 2017;88:222–31. https://doi.org/10.23750/abm.v88i2.6664.
Su H, Liu Y, Yin D, et al. Neuroplastic changes in resting-state functional connectivity after rTMS intervention for methamphetamine craving. Neuropharmacology. 2020;175: 108177. https://doi.org/10.1016/j.neuropharm.2020.108177.
Siefried KJ, Acheson LS, Litzeris N, Ezard N. Pharmacological treatment of methamphetamine/amphetamine dependence: a systematic review. CNS Drugs. 2020;34:337–65. https://doi.org/10.1007/s40263-020-00711-x.
Farrell M, Martin NK, Stockings E, et al. Responding to global stimulant use: challenges and opportunities. Lancet. 2019;394:1652–67. https://doi.org/10.1016/S0140-6736(19)32230-5.
Jerin SJF, Kimura-kataoka K, Fujihara J, et al. Two fatal cases of caffeine poisoning. Shimane J Med Sci. 2020;37:103–7.
Magdalan J, Zawadzki M, Skowronek R, et al. Nonfatal and fatal intoxications with pure caffeine—report of three different cases. Forensic Sci Med Pathol. 2017;13:355–8. https://doi.org/10.1007/s12024-017-9885-2.
Ymamoto T, Yoshizawa K, Kubo S, et al. Autopsy report for a caffeine intoxication case and review of the current literature. J toxicol Pathol. 2015;28:33–6. https://doi.org/10.1293/tox.2014-0044.
Sajja RK, Rahman S, Cucullo L. Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J Cereb Blood Flow Metab. 2016;36:539–54. https://doi.org/10.1177/0271678X15616978.
Polesskaya O, Silva J, Sanfilippo C, et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011;1373:91–100. https://doi.org/10.1016/j.brainres.2010.12.017.
Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocrit Care. 2009;10:295–305. https://doi.org/10.1007/s12028-008-9177-5.
Tominaga M, Michiue T, Ishikawa T, Inamori-Kawamoto O, Oritani S, Maeda H. Evaluation of postmortem drug concentrations in cerebrospinal fluid compared with blood and pericardial fluid. Forensic Sci Int. 2015;254:118–25. https://doi.org/10.1016/j.forsciint.2015.07.005.
Tominaga M, Michiue T, Inamori-Kawamoto O, et al. Efficacy of drug screening in forensic autopsy: retrospective investigation of routine toxicological findings. Leg Med. 2015;17:172–6. https://doi.org/10.1016/j.legalmed.2015.01.001.
Inamori-Kawamoto O, Ishikawa T, Michiue T, et al. Possible application of CT morphometry of the calcaneus and talus in forensic anthropological identification. Int J Legal Med. 2016;130:575–85. https://doi.org/10.1007/s00414-015-1258-3.
Takahashi N, Satou C, Higuchi T, Shiotani M, Maeda H, Hirose Y. Quantitative analysis of brain edema and swelling on early postmortem computed tomography: comparison with antemortem computed tomography. Jpn J Radiol. 2010;28:349–54. https://doi.org/10.1007/s11604-010-0430-4.
Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–7. https://doi.org/10.1161/01.str.31.9.2163.
Shirota G, Gonoi W, Ishida M, et al. Brain swelling and loss of gray and white matter differentiation in human postmortem cases by computed tomography. PLoS ONE. 2015;10: e0143848. https://doi.org/10.1371/journal.pone.0143848.
Winklhofer S, Surer E, Ampanozi G, et al. Post-mortem whole body computed tomography of opioid (heroin and methadone) fatalities: frequent findings and comparison to autopsy. Eur Radiol. 2014;24:1276–82. https://doi.org/10.1007/s00330-014-3128-7.
Zedler B, Flaig B, Ackermann H, Parzeller M, Bratzke H. Brain weight in completed suicide and other cases of death-comparison of recent and previous studies. Int J Legal Med. 2014;128:295–301. https://doi.org/10.1007/s00414-013-0913-9.
Soltani N, Soltani Z, Khaksari M, Ebrahimi G, Hajmohammmadi M, Iranpour M. The changes of brain edema and neurological outcome, and the probable mechanisms in diffuse traumatic brain injury induced in rats with the history of exercise. Cell Mol Neurobiol. 2020;40:555–67. https://doi.org/10.1007/s10571-019-00753-w.
Shida A, Ikeda T, Tani N, et al. Cortisol levels after cold exposure are ndependent of adrenocorticotropic hormone stimulation. PLoS ONE. 2020;15: e0218910. https://doi.org/10.1371/journal.pone.0218910.
Tanaka F, Tominaga K, Fujikawa Y, et al. Concentration of glia cell line-derived neurotropic factor positively correlates with symptoms in functional dyspepsia. Dig Dis Sci. 2016;61:3478–85. https://doi.org/10.1007/s10620-016-4329-5.
Von Rüden EL, Gualtieri F, Schönhoff K, et al. Molecular alterations of the TLR4-signaling cascade in canine epilepsy. BMC Vet Res. 2020;16:18. https://doi.org/10.1186/s12917-020-2241-x.
Dong Y, Gong W, Hua Z, et al. Combination of rapamycin and MK-2206 induced cell death via autophagy and necroptosis in MYCN-amplified neuroblastoma cell lines. Front Pharmacol. 2020;11:31. https://doi.org/10.3389/fphar.2020.00031.
Min X, Zeng X, Zhao W, et al. Cryptotanshinone protects dextran sulfate sodium-induced experimental ulcerative colitis in mice by inhibiting intestinal inflammation. Phytother Res. 2020;34:2639–48. https://doi.org/10.1002/ptr.6693.
Hishino Y, Nakata K, Hoshino S, et al. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195:495–505. https://doi.org/10.1084/jem.20011614.
Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–5.
Ikeda T, Tani N, Watanabe M, et al. Evaluation of cytokines and structural proteins to analyze the pathology of febrile central nervous system disease. Leg Med. 2021;51: 101864. https://doi.org/10.1016/j.legalmed.2021.101864.
Liang Z, Yin P, Zhao L. Effects of combined toxicity of methamphetamine and ketamine on apoptosis, oxidative stress and genotoxicity in HepG2 cells. Food Chem Toxicol. 2019;132: 110653. https://doi.org/10.1016/j.fct.2019.110653.
Kanthasamy A, Anantharam V, Ali SF, Kanthasamy AG. Methamphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin-D in methamphetamine-induced apoptotic cell death. Ann NY Acad Sci. 2006;1074:234–44. https://doi.org/10.1196/annals.1369.022.
Badisa RB, Wiley C, Randell K, et al. Identification of cytotoxic markers in methamphetamine treated rat C6 astroglia-like cells. Sci Rep. 2019;9:9412. https://doi.org/10.1038/s41598-019-45845-1.
Levy AD, Harcke HT, Mallak CT. Postmortem imaging MDCT features of postmortem change and decomposition. Am J Forensic Med Pathol. 2010;31:12–7. https://doi.org/10.1097/PAF.0b013e3181c65e1a.
Nishiyama Y, Kanayama H, Mori H, et al. Whole brain analysis of postmortem density changes of grey and white matter on computed tomography by statistical parametric mapping. Eur Radiol. 2017;27:2317–25. https://doi.org/10.1007/s00330-016-4633-7.
Randall Baselt C. Disposition of toxic drugs and chemicals in man. 9th ed. California: Biomedical Publications; 2011. p. 591–4.
Dietrich M, Koska V, Hecker C, et al. Protective effects of 4-aminopyridine in experimental optic neuritis and multiple sclerosis. Brain. 2020;143:1127–42. https://doi.org/10.1093/brain/awaa062.
Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. https://doi.org/10.1016/j.pneurobio.2005.04.003.
Seiden LS, Sabol KE. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. https://doi.org/10.1146/annurev.pa.33.040193.003231.
Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. https://doi.org/10.1111/j.1471-4159.2007.05196.x.
Randall Baselt C. Disposition of toxic drugs and chemicals in man. 9th ed. California: Biomedical Publications; 2011. p. 63–4.
Wang J, Kim D. Activation of voltage-dependent K + channels strongly limits hypoxia-induced elevation of [Ca 2+] i in rat carotid body glomus cells. J Physiol. 2018;596:3119–36. https://doi.org/10.1113/JP275275.
Zhao W, Zhao YL, Liu M, et al. Possible repair mechanisms of renin-angiotensin system inhibitors, matrix metalloproteinase-9 inhibitors and protein hormones on methamphetamine-induced neurotoxicity. Mol Biol Rep. 2021;48:7509–16. https://doi.org/10.1007/s11033-021-06741-y.
Gonçalves J, Leitão RA, Higuera-Matas A, et al. Extended-access methamphetamine self-administration elicits neuroinflammatory response along with blood-brain barrier breakdown. Brain Behav Immun. 2017;62:306–17. https://doi.org/10.1016/j.bbi.2017.02.017.
Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. https://doi.org/10.1038/nchembio.83.
Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23. https://doi.org/10.1016/j.cell.2008.10.044.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. https://doi.org/10.1038/nature14191.
Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. https://doi.org/10.1038/nchembio711.
Re DB, Verche VL, Yu C, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8. https://doi.org/10.1016/j.neuron.2014.01.011.
Jouan-Lanhouet S, Riquet F, Duprez L, Berghe TV, Takahashi N, Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol. 2014;35:2–13. https://doi.org/10.1016/j.semcdb.2014.08.010.
Adachi N. Cerebral ischemia and histamine. Folia Pharmacol Jpn. 2002;120:215–21.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was evaluated by the Independent Ethics Committee of the Osaka City University Graduate School of Medicine. Informed consent form opt-out for the autopsy data analysis was approved by the Independent Ethics Committee of Osaka City University Graduate School of Medicine (authorization no. 2001). The Ethics Committee approval for the study is valid for the period from April 5, 2011, to March 31, 2038.
Informed consent
The authors complied with the ethics approval (authorization no.2001) that was obtained from the Independent Ethics Committee of the Osaka City University Graduate School of Medicine regarding informed consent for publication of the case details.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ikeda-Murakami, K., Ikeda, T., Watanabe, M. et al. Central nervous system stimulants promote nerve cell death under continuous hypoxia. Human Cell 35, 1391–1407 (2022). https://doi.org/10.1007/s13577-022-00734-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00734-0