Abstract
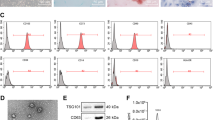
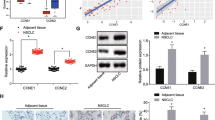
Mesenchymal stem cell (MSCs)-derived exosomes have been frequently used as useful tools in disease control. This research aimed to study the function of MSC-derived exosomes (Exo) in the stemness of cancer stem cells (CSCs) of hepatocellular carcinoma (HCC) and the molecular mechanism. Exo from the procured human bone marrow-MSCs were extracted and identified. CSCs from HCC cell lines were collected. The CSCs were treated with Exo, and then the proliferation, migration, invasion, angiogenesis-stimulating and self-renewal abilities of the Hep3B-CSCs and HuH7-CSCs were significantly reduced. C5orf66-AS1 was found as the most upregulated long noncoding RNAs (lncRNAs) in CSCs after Exo treatment. The integrated bioinformatic analyses and luciferase assays suggested that C5orf66-AS1 upregulated DUSP1 expression through sequestering microRNA-127-3p (miR-127-3p). Either artificial overexpression of miR-127-3p or silencing of DUSP1 blocked the inhibitory functions of Exo in the CSCs. DUSP1 inhibition increased the phosphorylation of ERK. Similar results were reproduced in vivo where Exo reduced the growth of xenograft formed by CSCs in nude mice, and this reduction was blocked upon miR-127-3p overexpression or DUSP1 silencing. To conclude, this research reported that MSC-derived Exo block malignant behaviors of HCC-sourced CSCs through a C5orf66-AS1/miR-127-3p/DUSP1/ERK axis.









Similar content being viewed by others
Data availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ATCC:
-
American type culture collection
- BCA:
-
Bicinchoninic acid
- BCLC:
-
Barcelona clinic liver cancer
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- CCK-8:
-
Cell counting kit-8
- CSCs:
-
Cancer stem cells
- CM:
-
Conditioned media
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DUSP1:
-
Dual-specificity phosphatase 1
- Exo:
-
Exosomes
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GEPIA:
-
Gene expression profiling interactive analysis
- HCC:
-
Hepatocellular carcinoma
- HUVECs:
-
Human umbilical vein endothelial cells
- IgG:
-
Immunoglobulin G
- IHC:
-
Immunohistochemical staining
- LDA:
-
Limited-dilution assay
- lncRNA:
-
Long noncoding RNA
- mean ± SD:
-
Mean ± standard deviation
- MT:
-
Mutant type
- MTT:
-
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- NC:
-
Negative control
- NIH:
-
National Institutes of Health
- NTA:
-
Nanoparticle tracking analysis
- OD:
-
Optical density
- PBS:
-
Phosphate-buffered saline
- PFA:
-
Paraformaldehyde
- RIPA:
-
Radio-immunoprecipitation assay
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- TEM:
-
Transmission electron microscope
- WT:
-
Wild type
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Karadag Soylu N. Update on hepatocellular carcinoma: a brief review from pathologist standpoint. J Gastrointest Cancer. 2020;51(4):1176–86. https://doi.org/10.1007/s12029-020-00499-5.
Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018;412:283–8. https://doi.org/10.1016/j.canlet.2017.10.008.
Zhang X, Xu X, Ge G, Zang X, Shao M, Zou S, Zhang Y, Mao Z, Zhang J, Mao F, Qian H, Xu W. miR498 inhibits the growth and metastasis of liver cancer by targeting ZEB2. Oncol Rep. 2019;41(3):1638–48. https://doi.org/10.3892/or.2018.6948.
Liu G, Luo Q, Li H, Liu Q, Ju Y, Song G. Increased oxidative phosphorylation is required for stemness maintenance in liver cancer stem cells from hepatocellular carcinoma cell line HCCLM3 cells. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155276.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84. https://doi.org/10.1038/nrc1590.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–3. https://doi.org/10.1038/nature07733.
Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–92. https://doi.org/10.1038/s41409-019-0616-z.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. https://doi.org/10.1186/1478-811X-9-12.
Lin W, Huang L, Li Y, Fang B, Li G, Chen L, Xu L. Mesenchymal stem cells and cancer: clinical challenges and opportunities. Biomed Res Int. 2019;2019:2820853. https://doi.org/10.1155/2019/2820853.
Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomedicine. 2019;14:2847–59. https://doi.org/10.2147/IJN.S200036.
Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272: 119232. https://doi.org/10.1016/j.lfs.2021.119232.
Lv H, Lv G, Han Q, Yang W, Wang H. Noncoding RNAs in liver cancer stem cells: the big impact of little things. Cancer Lett. 2018;418:51–63. https://doi.org/10.1016/j.canlet.2018.01.001.
Liu AY, Cai Y, Mao Y, Lin Y, Zheng H, Wu T, Huang Y, Fang X, Lin S, Feng Q, Huang Z, Yang T, Luo Q, Ouyang G. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis. 2014;35(3):537–45. https://doi.org/10.1093/carcin/bgt364.
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–66. https://doi.org/10.1016/j.ccr.2008.01.013.
Ran RZ, Chen J, Cui LJ, Lin XL, Fan MM, Cong ZZ, Zhang H, Tan WF, Zhang GQ, Zhang YJ. miR-194 inhibits liver cancer stem cell expansion by regulating RAC1 pathway. Exp Cell Res. 2019;378(1):66–75. https://doi.org/10.1016/j.yexcr.2019.03.007.
Liao B, Zhou H, Liang H, Li C. Regulation of ERK and AKT pathways by hepatitis B virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int J Oncol. 2017;51(5):1449–59. https://doi.org/10.3892/ijo.2017.4126.
de Araujo FV, O’Valle F, Serrano-Saenz S, Anderson P, Andres E, Lopez-Penalver J, Tovar I, Nieto A, Santos A, Martin F, Exposito J, Oliver FJ, de Almodovar JMR. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 2018;17(1):122. https://doi.org/10.1186/s12943-018-0867-0.
Cao Y, Ji C, Lu L. Mesenchymal stem cell therapy for liver fibrosis/cirrhosis. Ann Transl Med. 2020;8(8):562. https://doi.org/10.21037/atm.2020.02.119.
Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363(1):114–20. https://doi.org/10.1016/j.yexcr.2017.12.021.
Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 2018;26(7):1610–23. https://doi.org/10.1016/j.ymthe.2018.05.009.
Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18071450.
Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer’s disease. Int Immunol. 2017;29(1):11–9. https://doi.org/10.1093/intimm/dxx002.
Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6): e346. https://doi.org/10.1038/emm.2017.63.
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. https://doi.org/10.1126/scisignal.2005231.
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013;8(12): e84256. https://doi.org/10.1371/journal.pone.0084256.
Mandal S, Arfuso F, Sethi G, Dharmarajan A, Warrier S. Encapsulated human mesenchymal stem cells (eMSCs) as a novel anti-cancer agent targeting breast cancer stem cells: development of 3D primed therapeutic MSCs. Int J Biochem Cell Biol. 2019;110:59–69. https://doi.org/10.1016/j.biocel.2019.02.001.
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, Zhao L, Chen Z, Zheng M, Liu Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4. https://doi.org/10.1186/s13046-019-1512-5.
Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. https://doi.org/10.1186/s13045-015-0220-7.
Zhou Q, Li H, Jing J, Yuan Y, Sun L. Evaluation of C5orf66-AS1 as a potential biomarker for predicting early gastric cancer and its role in gastric carcinogenesis. Onco Targets Ther. 2020;13:2795–805. https://doi.org/10.2147/OTT.S239965.
Lu T, Liu H, You G. Long non-coding RNA C5orf66-AS1 prevents oral squamous cell carcinoma through inhibiting cell growth and metastasis. Int J Mol Med. 2018;42(6):3291–9. https://doi.org/10.3892/ijmm.2018.3913.
Yu G, Li C, Xie W, Wang Z, Gao H, Cao L, Hao L, Zhang Y. Long non-coding RNA C5orf66-AS1 is downregulated in pituitary null cell adenomas and is associated with their invasiveness. Oncol Rep. 2017;38(2):1140–8. https://doi.org/10.3892/or.2017.5739.
Jiang H, Hua D, Zhang J, Lan Q, Huang Q, Yoon JG, Han X, Li L, Foltz G, Zheng S, Lin B. MicroRNA-127-3p promotes glioblastoma cell migration and invasion by targeting the tumor-suppressor gene SEPT7. Oncol Rep. 2014;31(5):2261–9. https://doi.org/10.3892/or.2014.3055.
Ji L, Zhu ZN, He CJ, Shen X. MiR-127-3p targets KIF3B to inhibit the development of oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2019;23(2):630–40. https://doi.org/10.26355/eurrev_201901_16877.
Livingstone MC, Johnson NM, Roebuck BD, Kensler TW, Groopman JD. Profound changes in miRNA expression during cancer initiation by aflatoxin B1 and their abrogation by the chemopreventive triterpenoid CDDO-Im. Mol Carcinog. 2017;56(11):2382–90. https://doi.org/10.1002/mc.22635.
Hao PP, Li H, Lee MJ, Wang YP, Kim JH, Yu GR, Lee SY, Leem SH, Jang KY, Kim DG. Disruption of a regulatory loop between DUSP1 and p53 contributes to hepatocellular carcinoma development and progression. J Hepatol. 2015;62(6):1278–86. https://doi.org/10.1016/j.jhep.2014.12.033.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. https://doi.org/10.1038/sj.onc.1210421.
Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–42. https://doi.org/10.1038/nrd4281.
Jang HJ, Bak Y, Pham TH, Kwon SB, Kim BY, Hong J, Yoon DY. STK899704 inhibits stemness of cancer stem cells and migration via the FAK-MEK-ERK pathway in HT29 cells. BMB Rep. 2018;51(11):596–601.
Wei F, Zhang T, Deng SC, Wei JC, Yang P, Wang Q, Chen ZP, Li WL, Chen HC, Hu H, Cao J. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019;450:1–13. https://doi.org/10.1016/j.canlet.2019.02.022.
Guo J, Guo M, Zheng J. Inhibition of bone morphogenetic Protein 2 suppresses the stemness maintenance of cancer stem cells in hepatocellular carcinoma via the MAPK/ERK pathway. Cancer Manag Res. 2021;13:773–85. https://doi.org/10.2147/CMAR.S281969.
Tsai CF, Hsieh TH, Lee JN, Hsu CY, Wang YC, Kuo KK, Wu HL, Chiu CC, Tsai EM, Kuo PL. Curcumin suppresses phthalate-induced metastasis and the proportion of cancer stem cell (CSC)-like cells via the inhibition of AhR/ERK/SK1 signaling in hepatocellular carcinoma. J Agric Food Chem. 2015;63(48):10388–98. https://doi.org/10.1021/acs.jafc.5b04415.
Funding
This work was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2017D01C306).
Author information
Authors and Affiliations
Contributions
HG and CY: conceptualization, methodology, software, and writing—original draft preparation; LW: data curation; JJL: visualization and investigation; ZQZ: software and validation; DZG and HJW: conceptualization, writing—reviewing & editing, and writing—original draft preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Signed informed consent was acquired from each participant. The research was approved by the Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University (Approval number: K201807-05) and performed according to the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, H., Yan, C., Wan, H. et al. Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Human Cell 34, 1812–1829 (2021). https://doi.org/10.1007/s13577-021-00599-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00599-9