Abstract
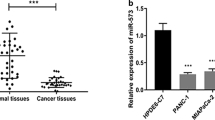
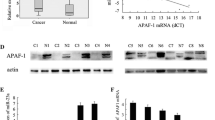
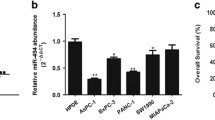
Pancreatic cancer is a malignant disease with poor prognosis. Emerging evidences have showed that miR-597-5p is closely related to tumor development. However, the functional roles of miR-597-5p in pancreatic cancer remain unknown. This study aimed to investigate the expression of miR-597-5p in pancreatic cancer tissues and cells, and explored its regulatory mechanism during pancreatic cancer progression. Pancreatic cancer and adjacent tissues were obtained to detect the expression of miR-597-5p by RT-qPCR. Cell growth, apoptosis, and related protein expression were, respectively, tested by CCK-8 assay, cell formation, wound healing, Transwell assay, flow cytometry, and western blotting. Finally, the pancreatic cancer mice model was constructed. In vitro and in vivo results showed that miR-597-5p expression was down-regulated in pancreatic cancer tissues and cell lines, and increased the overall survival of pancreatic cancer patients. Moreover, miR-597-5p decreased pancreatic cancer cell viability, reduced relative wound width, suppressed colony formation and decreased invasive cell number, as well as reduced the expression of proliferating cell nuclear antigen (PCNA), Ki67, Cyclin D1, N-cad, and Bcl-2. Meanwhile, it increased pancreatic cancer cell apoptosis and the expression of E-cad, cleaved caspase 3, and Bax. The dual-luciferase reporter assay confirmed miR-597-5p could directly target e-twenty six like-1 (ELK1) oncogene. The reduction of cell growth and the induction of cell apoptosis induced by miR-597-5p were reversed by ELK1. In addition, miR-597-5p inhibited the growth of pancreatic cancer in vivo. This study demonstrated that miR-597-5p may be a novel suppressor of pancreatic cancer. It inhibits pancreatic cancer cell growth and promotes apoptosis by the down-regulation of ELK1 in vitro and in vivo.






Similar content being viewed by others
References
Chua YJ, Cunningham D. Adjuvant treatment for resectable pancreatic cancer. J Clin Oncol. 2005;23(20):4532–7. https://doi.org/10.1200/jco.2005.17.954.
Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomark Prev. 2001;10(5):429–37.
Li B-Q, Wang L, Li J, Zhou L, Zhang T-P, Guo J-C, et al. Surgeons’ knowledge regarding the diagnosis and management of pancreatic cancer in China: a cross-sectional study. BMC Health Serv Res. 2017;17(1):395. https://doi.org/10.1186/s12913-017-2345-6.
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. https://doi.org/10.1038/nature09515.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Beger HG, Rau B, Gansauge F, Leder G, Schwarz M, Poch B. Pancreatic cancer–low survival rates. Dtsch Arzteblatt Int. 2008;105(14):255–62. https://doi.org/10.3238/arztebl.2008.0255.
van Vuurden DG, Hulleman E, Irandoust M, Biesmann D, van den Berg T, Aronica E, et al. SIRPαα is transcriptionally downregulated by epigenetic silencing in medulloblastoma. J Mol Clin Med. 2018;1(3):157–68.
Li MF, Li J, Ding XF, Cheng SY. MicroRNA and cancer. Aaps J. 2010;12:309–317. https://doi.org/10.1208/s12248-010-9194-0
He J, Mai J, Li Y, Chen L, Xu H, Zhu X, et al. miR-597 inhibits breast cancer cell proliferation, migration and invasion through FOSL2. Oncol Rep. 2017;37(5):2672–8. https://doi.org/10.3892/or.2017.5558.
Frampton AE, Castellano L, Colombo T, Giovannetti E, Krell J, Jacob J, et al. MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology. 2014;146(1):268–77.e18. https://doi.org/10.1053/j.gastro.2013.10.010.
Felix TF, Lopez Lapa RM, de Carvalho M, Bertoni N, Tokar T, Oliveira RA, et al. MicroRNA modulated networks of adaptive and innate immune response in pancreatic ductal adenocarcinoma. PLoS ONE. 2019;14(5):e0217421. https://doi.org/10.1371/journal.pone.0217421.
Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem. 2011;80:437–71. https://doi.org/10.1146/annurev.biochem.79.081507.103945.
Ahmad A, Zhang W, Wu M, Tan S, Zhu TJG. Tumor-suppressive miRNA-135a inhibits breast cancer cell proliferation by targeting ELK1 and ELK3 oncogenes. Genes Genom. 2018;40(3):243–51. https://doi.org/10.1007/s13258-017-0624-6.
National Research Council Institute for Laboratory Animal R. Guide for the Care and Use of Laboratory Animals. Washington: National Academies Press; 1996.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, CA). 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Wang Y, Kuramitsu Y, Yoshino S, Takashima M, Zhang X, Ueno T, et al. Screening for serological biomarkers of pancreatic cancer by two-dimensional electrophoresis and liquid chromatography-tandem mass spectrometry. Oncol Rep. 2011;26(1):287–92. https://doi.org/10.3892/or.2011.1278.
Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Can Res. 2010;70(14):6015–25. https://doi.org/10.1158/0008-5472.can-09-4531.
Zoller M. Pancreatic cancer diagnosis by free and exosomal miRNA. World J Gastrointest Pathophysiol. 2013;4(4):74–90. https://doi.org/10.4291/wjgp.v4.i4.74.
Zhao G, Wang B, Liu Y, Zhang JG, Deng SC, Qin Q, et al. miRNA-141, downregulated in pancreatic cancer, inhibits cell proliferation and invasion by directly targeting MAP4K4. Mol Cancer Ther. 2013;12(11):2569–80. https://doi.org/10.1158/1535-7163.mct-13-0296.
Zhang XY, Liu DJ, Yuan RB, Zhang DH, Li SR, Zhang SH, et al. Low expression of miR-597 is correlated with tumor stage and poor outcome in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22(2):456–60. https://doi.org/10.26355/eurrev_201801_14195.
Xie L, Jiang T, Cheng A, Zhang T, Huang P, Li P, et al. MiR-597 targeting 14–3-3sigma enhances cellular invasion and EMT in nasopharyngeal carcinoma cells. Curr Mol Pharmacol. 2019;12(2):105–14. https://doi.org/10.2174/1874467212666181218113930.
Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. https://doi.org/10.1101/gad.1640608.
Janknecht R, Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992;20(13):3317–24. https://doi.org/10.1093/nar/20.13.3317.
Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991;354(6354):531–4. https://doi.org/10.1038/354531a0.
Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73(2):381–93. https://doi.org/10.1016/0092-8674(93)90237-k.
Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20(12):4563–72.
Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 1998;273(47):31327–36. https://doi.org/10.1074/jbc.273.47.31327.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SZL and XLL conceived and designed the experiments, XHX analyzed and interpreted the results of the experiments, and LW performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests, and all authors should confirm its accuracy.
Ethics approval and consent to participate
All animal experiments were followed by the Guide for the Care and Use of Laboratory Animals and approved by the Ethical Committee of Hainan General Hospital. The specimens of pancreatic cancer and adjacent tissues were collected from the patients who were diagnosed with pancreatic cancer in Hepatobiliary Surgery of Hainan General Hospital. The informed consents for each patient were obtained and the procedures were approved by the Ethics Committee of Hainan General Hospital (Approval no.[2019]49).
Informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Li, X., Xing, X. et al. miR-597-5p inhibits cell growth and promotes cell apoptosis by targeting ELK1 in pancreatic cancer. Human Cell 33, 1165–1175 (2020). https://doi.org/10.1007/s13577-020-00395-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00395-x