Abstract
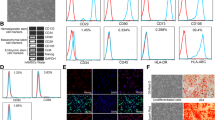
Migration and homing are known as critical steps toward regeneration of damaged tissues via cell therapies. Among various cellular sources of stem cells, the umbilical cord has been thus recognized as an interesting one endowed with high benefits. Accordingly, the main objective of the present study was to determine whether monophosphoryl lipid A (MPLA) or supernatant of Lactobacillus acidophilus (SLA) could increase migration of human umbilical cord mesenchymal stem cells (hUMSCs) toward acellular foreskin or not. In this study, the hUMSCs were isolated and cultured through acellular MPLA- or SLA-treated foreskin. Expression of some migration genes (i.e., VCAM-1, MMP-2, VLA-4, CXCR-4, and VEGF) was also investigated using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Moreover; vimentin, cytokeratin 5 (CK5), and matrix metalloproteinases-2 (MMP-2) were detected via immunohistochemistry (IHC) analysis. The hUMSCs in the presence of MPLA- or SLA-treated foreskin showed more tissue tropism compared with those in the control group. Besides, the scanning electron microscopy (SEM) results established that the hUMSCs had more migratory activity in the presence of MPLA- or SLA-treated foreskin than the untreated one. The IHC analysis results correspondingly indicated that expression of vimentin, CK5, and MMP-2 proteins had augmented in both treatments compared with those in the control group. It was concluded that MPLA had revealed more prominent results than SLA, even though both treatments could be regarded as inducing factors in migration. Ultimately, it was suggested to introduce the use of MPLA and probiotic components as a promising approach to improve therapies in regenerative medicine.









Similar content being viewed by others
References
Dong F, Caplan AI. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transpl. 2012;17(6):670–4.
Yun S, Shin T-H, Lee J-H, Cho MH, Kim I-S, Kim J-W, et al. Design of magnetically labeled cells (mag-cells) for in vivo control of stem cell migration and differentiation. Nano Lett. 2018;18(2):838–45.
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–8.
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16(3):571–9.
Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33(31):7849–57.
Bartold P, Gronthos S, Haynes D, Ivanovski S. Mesenchymal stem cells and biologic factors leading to bone formation. J Clin Periodontol. 2019;46(21):12–32.
Kang J, Fan W, Deng Q, He H, Huang F. Stem cells from the apical papilla: a promising source for stem cell-based therapy. BioMed Res Int. 2019;5:1–8. https://doi.org/10.1155/2019/6104738.
Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20(121):e33.
Azzopardi JI, Blundell R. Umbilical cord stem cells. Stem Cell Discov. 2018;8(01):1.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
Percer B. Umbilical cord blood banking: helping parents make informed choices. Nurs Women Health. 2009;13(3):216–23.
Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):893–908.
Gonzalez-Ryan L, Van Syckle K, Coyne KD, Glover N. Umbilical cord blood banking: procedural ad ethical concerns for this new birth option. Pediat Nurs. 2000;26(1):105.
Ravi M, Paramesh V, Kaviya S, Anuradha E, Solomon FP. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230(1):16–26.
Zhou P, Liu Z, Li X, Zhang B, Wang X, Lan J, et al. Migration ability and Toll-like receptor expression of human mesenchymal stem cells improves significantly after three-dimensional culture. Biochem Biophys Res Commun. 2017;491(2):323–8.
Atala A. Regenerative medicine strategies. J Pediatr Surg. 2012;47(1):17–28.
Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013. https://doi.org/10.1155/2013/130763.
Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–45.
Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79(9):3576–87.
Myers KR, Truchot A, Ward J, Hudson Y, Ulrich J. A critical determinant of lipid A endotoxic activity. Amsterdam: Elsevier Science; 1990. p. 145–56.
Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65(8):3239–47.
Ulrich J, Masihi K, Lange W. Mechanisms of nonspecific resistance to microbial infections induced by trehalose dimycolate (TDM) and monophosphoryl lipid A (MPL). Adv Biosci. 1988;68:167–78.
Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol. 2011;45:S133–8.
Halper J, Leshin L, Lewis S, Li W. Wound healing and angiogenic properties of supernatants from Lactobacillus cultures. Exp Biol Med. 2003;228(11):1329–37.
Dehkordi MB, Madjd Z, Chaleshtori MH, Meshkani R, Nikfarjam L, Kajbafzadeh A-M. A simple, rapid, and efficient method for isolating mesenchymal stem cells from the entire umbilical cord. Cell Transplant. 2016;25(7):1287–97.
Saberian M, Shahidi Delshad E, Naji T, Samadikuchaksaraei A. Comparing the effect of Lactobacillus acidophilus and MRS medium on mesenchymal stem cells proliferation. J Payavard Salamat. 2015;9(3):276–87.
Ivaska J, Pallari H-M, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313(10):2050–62. https://doi.org/10.1016/j.yexcr.2007.03.040.
Palmieri C, Story M, Lean F, Akter S, Grieco V, De Marzo AM. Diagnostic utility of cytokeratin-5 for the identification of proliferative inflammatory atrophy in the canine prostate. J Comp Pathol. 2018;158:1–5.
Hingorani DV, Lippert CN, Crisp JL, Savariar EN, Hasselmann JPC, Kuo C, et al. Impact of MMP-2 and MMP-9 activation on wound healing, tumor growth and RACPP cleavage. biorxiv. 2018. https://doi.org/10.1101/327791.
Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24(5):1254–64.
Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34 + cells in response to chemokines. Exp Hematol. 2000;28(11):1274–85.
Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32(9):483–92.
Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–44.
Shojaeian A, Mehri-Ghahfarrokhi A, Banitalebi-Dehkordi M. Migration gene expression of human umbilical cord mesenchymal stem cells: a comparison between monophosphoryl lipid A and supernatant of Lactobacillus acidophilus. Int J Mol Cell Med. 2019;8(2).
Preidis GA, Saulnier DM, Blutt SE, Mistretta T-A, Riehle KP, Major AM, et al. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J. 2012;26(5):1960–9.
Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Najafabadi AH, et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J Control Release. 2018;282:131–9.
Ruchaud-Sparagano MH, Mills R, Scott J, Simpson AJ. MPLA inhibits release of cytotoxic mediators from human neutrophils while preserving efficient bacterial killing. Immunol Cell Biol. 2014;92(9):799–809.
Hu X, Liu R, Zhu N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology. 2013;218(12):1524–8. https://doi.org/10.1016/j.imbio.2013.05.006.
Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012. https://doi.org/10.1155/2012/342968.
Fu X, Han B, Cai S, Lei Y, Sun T, Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-α and its possible role in wound healing. Wound Repair Regenerat. 2009;17(2):185–91.
Chen M-S, Lin C-Y, Chiu Y-H, Chen C-P, Tsai P-J, Wang H-S. IL-1β-induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-coupled signaling pathway. Stem Cells Int. 2018. https://doi.org/10.1155/2018/3524759.
Li Y, Yu X, Lin S, Li X, Zhang S, Song Y-H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356(3):780–4.
Shi M, Li J, Liao L, Chen B, Li B, Chen L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904.
Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen–glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62.
Nickel W. Unconventional secretion: an extracellular trap for export of fibroblast growth factor 2. J Cell Sci. 2007;120(14):2295–9.
Mullen LM, Best SM, Brooks RA, Ghose S, Gwynne JH, Wardale J, et al. Binding and release characteristics of insulin-like growth factor-1 from a collagen–glycosaminoglycan scaffold. Tissue Eng Part C: Methods. 2010;16(6):1439–48.
Chavez-Munoz C, Nguyen KT, Xu W, Hong S-J, Mustoe TA, Galiano RD. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: engineering a stratified epidermis. PLoS One. 2013;8(12):e80587. https://doi.org/10.1371/journal.pone.0080587.
Toai TC, Thao HD, Gargiulo C, Thao NP, Thuy TTT, Tuan HM, et al. In vitro culture of Keratinocytes from human umbilical cord blood mesenchymal stem cells: the Saigonese culture. Cell Tissue Bank. 2011;12(2):125–33.
dos Santos JF, Borçari NR, da Silva Araújo M, Nunes VA. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: Towards an in vitro model of human epidermis. J Cell Biochem. 2019;120(8):13141–13155.
Secunda R, Vennila R, Mohanashankar A, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67(5):793–807.
Luo X, Gupta K, Ananthanarayanan A, Wang Z, Xia L, Li A, et al. Directed differentiation of adult liver derived mesenchymal like stem cells into functional hepatocytes. Sci Rep. 2018;8(1):2818.
Acknowledgements
The authors would like to express sincere thanks to the staffs of Cellular and Molecular Research Center of Basic Health Sciences Institute of Shahrekord University of Medical Sciences for their cooperation. This research project was extracted from a Ph.D. thesis and was supported by a deputy of research and technology of Shahrekord University of Medical Sciences (SKUMS) [Grant Number: 2757].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved by the Ethics and Clinical Studies Research Committee of SKUMS according to Helsinki Declaration (IR.SKUMS.REC.1397.80).
Informed consent
Informed consents were obtained from all mothers before surgery.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shojaeian, A., Mehri-Ghahfarrokhi, A. & Banitalebi-Dehkordi, M. Increased in vitro migration of human umbilical cord mesenchymal stem cells toward acellular foreskin treated with bacterial derivatives of monophosphoryl lipid A or supernatant of Lactobacillus acidophilus. Human Cell 33, 10–22 (2020). https://doi.org/10.1007/s13577-019-00308-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00308-7