Abstract
Stereotactic ablative radiotherapy (SABR), also named stereotactic body radiation therapy, entails the delivery of individual ablative doses of radiation to the tumor volume with a very steep dose gradient beyond the tumor volume using highly conformal techniques and has been used to treat primary and metastatic lung tumors with promising results achieved. As a result of the delivery of individual ablative doses to the lung, the resulting inflammatory (acute) and fibrotic (late) reactions are more intense, rendering interpretation of post-SABR imaging changes difficult. Different early and late injury patterns have been described based on computerized tomography (CT) by various groups of researchers. Although the data on post-SABR imaging changes are emerging, there are still challenges distinguishing between post-treatment changes and tumor progression. The necessity for evaluation based on serial imaging cannot be overemphasized. Controversies still exist as to whether CT or positron emission tomography (PET) should be used for follow-up. Since CT and PET are the most commonly used imaging modalities for follow-up after SABR, they will be the foci of this review article.
Similar content being viewed by others
Introduction
Stereotactic ablative radiotherapy (SABR), also named stereotactic body radiation therapy (SBRT), entails the delivery of individual ablative doses of radiation to the tumor volume with a very steep dose gradient beyond the tumor volume using highly conformal techniques [1]. SABR challenges the dogma of classical radiobiology for fractionated radiotherapy. The tumor and the adjacent normal lung parenchyma are likely damaged by the ablative doses of radiation, but if the damaged lung parenchyma is small in amount and if there is no eloquent tissue included in the prescribed isodose line, clinically significant toxicities will not develop [2]. To facilitate the safe delivery of SABR, proper equipment and training of personnel in the treatment team are essential. On-board imaging is required to verify the position of the tumor prior to treatment delivery.
Data from multiple retrospective studies and prospective clinical trials from across the world have demonstrated the safety and efficacy of SABR for the treatment of early-stage, predominantly medically inoperable, stage I non-small-cell lung carcinoma (NSCLC) [1, 2]. Most studies observed a local control rate of ~90% or higher. Different regimens have been used in clinical trials, but the treatment outcomes are comparable. SABR has also been used to treat lung oligometastases, and similarly, high rates of local control were reported in the literature although a substantial proportion of patients develop out-of-field recurrence [1].
Imaging changes after SABR for lung tumors can be confusing. Because ablative doses of radiation are given during SABR, the resulting inflammatory (acute) and fibrotic (late) reactions will be more intense [3]. As a result, it can be challenging to distinguish post-treatment changes from tumor progression. Computerized tomography (CT) and positron emission tomography (PET) are typically used for initial staging and for follow-up after SABR, and therefore, this review will focus on imaging changes on CT and PET.
Imaging changes on CT
Depending on the technique used, SABR typically utilizes multiple non-opposing and non-coplanar beans converging at the center of the lung tumor, generating a very steep dose gradient between the periphery of the planning treatment volume (PTV) and normal lung parenchyma [2]. The isodose distribution and radiobiologic effects of SABR are different from those of conventionally fractionated radiation therapy, and the radiographic changes typically conform to the shape of the PTV, which contains the gross tumor and a rim of normal lung parenchyma within the prescribed isodose line and possibly the shape of intermediate isodose shells.
Radiation injury after SABR can be classified as early and late, as in conventional radiotherapy. Early reaction usually occurs 1–6 months after treatment and late reaction beyond 6 months [3–5]. However, radiographic changes of normal lung parenchyma do not usually develop less than 3 months after SBRT. Early reaction such as radiation pneumonitis can progress to radiation fibrosis beyond 6 months after treatment, although patients may develop radiation fibrosis even without preceding radiation pneumonitis based on imaging findings [3–5].
Colleagues from the Mallinckrodt Institute of Radiology observed different acute and chronic patterns of changes after SABR, similar to those described by Kimura et al. from Hiroshima University, Japan, in 2006 [3–6]. Early changes are related to inflammation, and based on their observation, five different patterns have been observed [3–5]:
-
1.
Diffuse consolidation—diffuse, homogenous increase in lung parenchyma attenuation which obscures the margins of blood vessels and airway walls, completely filling the high-dose zone
-
2.
Diffuse ground-glass opacity—hazy increase in lung parenchymal attenuation completely filling the high-dose zone, with the margins of the vessels and airway wall preserved
-
3.
Patchy consolidation and ground-glass opacity—patchy areas of hazy and dense increase in lung attenuation, not completely filling the high-dose zone
-
4.
Patchy ground-glass opacity—patchy areas of hazy increase in the lung attenuation intermingled with normal lung parenchyma, not completely filling the high-dose zone; and
-
5.
No change
Late changes, related to fibrosis, include four different patterns [3–5]:
-
1.
Modified conventional pattern—consolidation, loss of volume, and bronchiectasis
-
2.
Mass-like pattern—focal consolidation in the region of the treated tumor
-
3.
Scar-like pattern—linear opacity in the area of the treated tumor associated with some volume loss; and
-
4.
No change
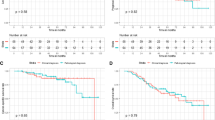
Guckenberger and colleagues observed the CT changes of 70 patients with 86 lung tumors (48 lung metastases and 38 primary NSCLC) treated with SBRT to dose regimens of 6–12.5 Gy × 3–8 fractions or 30 Gy × 1 based on 346 CT scans performed. Different early radiographic changes were observed, and they included ground-glass opacity, spotted condensation, and no change (76% within the first 6 weeks) [7]. Observed late radiographic changes, which developed 6–9 months after SABR, included streaky condensation, dense consolidation, and retraction [7]. The fibrotic changes tended to be confined to the 50–70% isodose lines. Continued changes in the volume and the appearance of the fibrotic areas were observed for years after SABR in some patients.
Other groups have also reported their observation of imaging changes after SABR for lung tumors. Investigators from the University of Alabama at Birmingham performed a dosimetric analysis of imaging changes after SABR for lung tumors to a dose of 30–60 Gy in 3 fractions and discovered that SABR-induced was very common and was well approximated by an 18-Gy isodose line based on a 3-fraction regimen [8]. Takeda et al. from Japan made an interesting observation that after SBRT for small peripheral lung tumors, early radiation-induced injury or radiation pneumonitis frequently started at the periphery of the PTV, but the CT changes eventually conformed to the PTV before late radiation-induced injury or radiation-induced fibrosis kicked in, typically more than 6 months after treatment [9]. The dense consolidation did not always precisely correspond to the PTV, and its shape and location might change dynamically during the first year. In another study by Takeda et al. where 50 patients with stage I NSCLC were treated with SABR to a dose of 50 Gy in 5 fractions, only 3 of 20 patients who had abnormal opacity were eventually found to have tumor recurrence [10]. Matsuo et al. from Kyoto University, Japan, evaluated the CT changes of 37 patients with 40 lung tumors treated with SBRT, using regimens of 10–12 Gy × 4–5 fractions and observed mass-like consolidation in 27 (68%) of 40 tumors at a median of 5 months [11]. A subsequent CT scan showed that only three (11%) of those truly represented local recurrence. One interesting finding was that on initial follow-up CT, there was no significant difference in the imaging findings comparing the group with fibrosis and the group with local recurrence, rendering evaluation of response at that time point difficult. None of the radiation-induced injury cases exhibited growth in size of the mass-like consolidation while all recurrence cases showed increase in size of the opacity [11]. Apart from increase in size of opacity after 12 months or longer, Kato and colleagues from Japan found that features such as a bulging margin, disappearance of air bronchograms, or appearance of pleural effusion were indicative of a local recurrence [12]. In a recent study from the Netherlands, Dahele et al. reviewed the CT of 61 patients with 68 lung tumors after SABR with at least 2 years of radiologic follow-up. All those patients had no evidence of local recurrence. Additional radiologic abnormalities, mostly scored as mild to moderate, occurred in 54% of lesions within 6 months and 99% after 36 months [13]. The median time to first development of radiologic abnormalities was 17 weeks. However, 25% of radiologic abnormalities occurred more than 1 year after SABR, and in 47% of lesions, the morphology or severity of changes continued to evolve more than 2 years after treatment [13]. Hof et al. from the University of Heidelberg discovered a significant correlation between dose level and the extent of normal tissue changes after single fraction SABR for lung tumors [14]. Palma et al. from the Netherlands showed that increased CT density was associated with a higher dose, increasing PTV size, and increasing time after SABR using regimens of 18 Gy × 3, 11 Gy × 5, and 7.5 Gy × 8. Increased densities appeared in areas receiving >6 Gy, were most prominent in areas receiving >20 Gy, and appeared to plateau at >40 Gy. The reduction in the air-filled fraction of the lung after treatment was up to 18% in areas receiving >36 Gy [15].
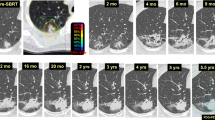
Based on our experience and data from the literature, it is sometimes difficult to distinguish between a mass-like consolidation and a tumor recurrence using CT imaging at one time point. Serial imaging studies and correlation of the SBRT isodose distribution and CT changes are important in order to determine whether the imaging changes are related to fibrosis or tumor recurrence. In general, fibrosis induced by ablative radiation doses tends to stabilize after 1–2 years. Figures 1 and 2 demonstrate the serial CT changes that occurred after SABR for medically inoperable NSCLC.
Imaging changes in PET
Fluorodeoxyglucose (FDG)-PET has been a useful tool for the evaluation of treatment response after conventional fractionated radiation therapy for NSCLC. It is unclear whether FDG-PET is equally useful in patients who received SABR for lung tumors. Data in the literature on the use of FDG-PET in the evaluation of post-SABR response are emerging [3]. One of the earliest studies includes a retrospective study from Indiana University, where 14% of patients treated with SABR for medically inoperable NSCLC in a prospective trial maintained a moderate standardized uptake value (SUV) (defined as higher than 2.5) 22–26 months after treatment and upon longer follow-up (42–49 months), remaining alive without evidence of disease recurrence [16]. In an Indiana University pilot trial of serial FDG-PET in patients with medically inoperable stage I non-small cell lung cancer treated with SABR, serial FDG-PET/CT was done before SBRT and at 2, 26, and 52 weeks after SABR. It was observed that patients with low pre-SBRT SUV were more likely to have an initial 2-week rise in SUV, and patients with high pre-SABR SUV frequently had a decline in SUV 2 weeks after treatment [17]. Six out of 13 patients had primary tumor maximum SUV of >3.5 at 12 months after SBRT but did without any evidence of local disease failure on subsequent follow-up. In a study from Kyoto University, Matsuo demonstrated that FDG uptake tended to be intense and well defined at early periods after SABR, especially within 6 months, and was faint and ill-defined at later periods. Moderate to intense FDG uptake on PET soon after SABR did not always represent a residual tumor [18]. Figures 3 and 4 showed the serial changes on FDG-PET after SABR.
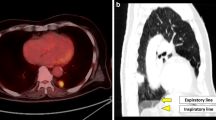
A 75-year-old gentleman with recurrent medically inoperable stage I NSCLC (pre-treatment SUV of 4.2) was treated with SABR to a dose of 45 Gy in 5 fractions. There was an increase of SUV after 7 months. The SUV then decreased to 4.7 at 10 months. Subsequent follow-up with CT did not show evidence of progression of fibrotic changes, and he had no evidence of disease 2.5 years after SABR
The right lung nodule was biopsy proven to be metastatic cervical carcinoma. Fused data from the PET/CT are shown (a). The lesion was treated with 12 Gy × 5 fractions and was reimaged at 4 months (b) and 13 months (c). Note the inflammation that was induced at 4 months and resolved at 13 months with lung parenchymal changes. Presumably, this showed evolution and resolution of acute radiation changes. The patient did not develop symptoms of radiation pneumonitis
Other studies have demonstrated good correlation between FDG-PET findings and response to treatment. Coon et al. found that after SABR for primary and recurrent NSCLC, and lung metastases, patients with stable disease, partial response, complete response, and progressive disease had a decrease in SUV of 28%, 48%, 94%, and 0.4%, respectively [19]. In a study by Fuss et al., 38 patients with stage I NSCLC were treated with SABR to doses ranging from 36 to 60 Gy in 3 fractions. Follow-up FDG-PET studies at an interval of 4–12 weeks were available in 30 patients. Changes in SUV were seen as early as 4 weeks, and there was a decrease of SUV to less than 3.0 in 28 patients at 12 weeks [20]. The remaining two patients without a decrease of SUV in the treated tumor developed local failure. Similar to the study by Fuss et al., Feigenberg et al. from Fox Chase Cancer Center found that a decrease of SUV on FDG-PET at 3 months after SABR strongly correlated with the local control in 18 patients treated for NSCLC. Sixty percent of patients with no decrease of SUV developed local failure 9 months to 1 year after post-SABR FDG-PET [21].
Where do we go from here?
Given the ablative nature of SABR, the inflammatory and fibrotic reactions in the lung parenchyma are more intense after treatment [3]. Based on the observations from multiple studies, CT findings of radiation-induced injury usually do not appear within the first 6 weeks after SABR [7]. Early radiation injury, caused by inflammation, starts to occur 2–6 months after treatment. Different patterns of changes on CT have been observed by various groups [3–7]. Late radiation injury starts to develop 6–9 months after treatment, and similar to early effects, different injury patterns can occur. One of the late injury patterns, mass-like consolidation, can occur in a significant proportion of patients after SABR and is frequently the source of confusion [3, 10, 11]. Most of the cases of mass-like consolidation represent dense fibrosis rather than tumor progression [3, 10]. The area of consolidation frequently does not correspond precisely to the treated PTV and may continue to shift in position within 1 year after treatment [9]. In general, fibrotic changes should stabilize after 1–2 years. However, continued changes may occur even after 2 years [13]. When there is growth of size of the mass consolidation, a bulging margin, loss of air bronchogram, or pleural effusion, tumor progression should be suspected [12].
In an attempt to more accurately evaluate response of lung tumors to SABR, FDG-PET, which demonstrates tumor activity based on glucose uptake, has been used as follow-up imaging. In general, a decrease of SUV correlates with tumor response and local control [19–21]. However, some studies show that SUV may transiently increase shortly after SABR, presumably due to inflammatory reaction [16–18]. Furthermore, moderate increase in SUV can occur 2 years or more after treatment in patients without evidence of tumor recurrence [16].
For both CT and FDG-PET, the need for follow-up using serial imaging cannot be overemphasized. Based on observations from the literature, controversies still exist as to whether FDG-PET is superior to CT for the follow-up of patients who receive SABR for lung tumors. Currently, Radiation Therapy and Oncology Group protocols of SABR for medically inoperable early-stage NSCLC predominantly are using CT to monitor tumor response, and FDG-PET is only obtained to look for FDG avidity similar to the initial tumor presentation if tumor enlargement occurs.
Conclusions
SABR has been demonstrated to be an effective and safe treatment for primary and metastatic lung tumors and has been widely adopted in practice. Although the data on post-SABR imaging changes are emerging, there are still challenges distinguishing between post-treatment changes and tumor progression. The necessity for evaluation based on serial imaging cannot be overemphasized. Controversies still exist as to whether CT or FDG-PET should be used for follow-up. More research is needed on diagnostic criteria for tumor progression versus post-treatment changes for both CT and PET. It is worthwhile to explore PET imaging techniques using tumor-specific radiotracers, which can potentially improve the positive predictive value for tumor recurrence.
References
Lo SS, Fakiris AJ, Chang EL et al (2010) Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 7:44–54
Lo SS, Fakiris AJ, Papiez L et al (2008) Stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Expert Rev Anticancer Ther 8:87–98
Lo SS, Teh BS, Wang JZ et al (2011) Imaging changes after stereotactic body radiation therapy for lung and liver tumors. Expert Rev Anticancer Ther 11:613–620
Linda A, Trovo M, Bradley JD (2011) Radiation injury of the lung after Stereotactic Body Radiation Therapy (SBRT) for lung cancer: a timeline and pattern of CT changes. Eur J Radiol 79:147–154
Trovo M, Linda A, El Naqa I et al (2010) Early and late lung radiographic injury following Stereotactic Body Radiation Therapy (SBRT). Lung Cancer 69:77–85
Kimura T, Matsuura K, Murakami Y et al (2006) CT appearance of radiation injury of the lung and clinical symptoms after Stereotactic Body Radiation Therapy (SBRT) for lung cancers: are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 66:483–491
Guckenberger M, Heilman K, Wulf J et al (2007) Pulmonary injury and tumor response after Stereotactic Body Radiotherapy (SBRT): results of a serial follow-up CT study. Radiother Oncol 85:435–442
Prendergast BM, Bonner JA, Popple RA et al (2011) Dosimetric analysis of imaging changes following pulmonary stereotactic body radiation therapy. J Med Imaging Radiat Oncol 55:90–96
Takeda T, Takeda A, Kunieda E et al (2004) Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR Am J Roentgenol 182:1123–1128
Takeda A, Kunieda E, Takeda T et al (2008) Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 70(4):1057–1065
Matsuo Y, Nagata Y, Mizowaki T et al (2007) Evaluation of mass-like consolidation after stereotactic body radiation therapy for lung tumors. Int J Clin Oncol 12:356–362
Kato S, Nambu A, Onishi H et al (2010) Computed tomography appearances of local recurrence after stereotactic body radiation therapy for stage I non-small-cell lung carcinoma. Jpn J Radiol 28:259–265
Dahele M, Palma D, Lagerwaard F et al (2011) Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol 6:1221–1228
Hof H, Zgoda J, Nill S et al (2010) Time- and dose-dependency of radiographic normal tissue changes of the lung after stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 77:1369–1374
Palma DA, van Sörnsen de Koste J, Verbakel WF et al (2011) Lung density changes after stereotactic radiotherapy: a quantitative analysis in 50 patients. Int J Radiat Oncol Biol Phys 81:974–978
Hoopes DJ, Tann M, Fletcher JW et al (2007) FDG-PET and Stereotactic Body Radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 56:229–234
Henderson MA, Hoopes DJ, Fletcher JW et al (2010) A pilot trial of serial 18f-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 76(3):789–795
Matsuo Y, Nakamoto Y, Nagata Y et al (2010) Characterization of FDG-PET images after stereotactic body radiation therapy for lung cancer. Radiother Oncol 97:200–204
Coon D, Gokhale AS, Burton SA et al (2008) Fractionated stereotactic body radiation therapy in the treatment of primary, recurrent, and metastatic lung tumors: the role of positron emission tomography/computed tomography-based treatment planning. Clin Lung Cancer 9:217–221
Fuss M, Salter BJ, Herman TS et al (2005) Metabolic PET imaging for Stereotactic Body Radiation Therapy (SBRT) planning and therapy response assessment of pulmonary malignancies. Int J Radiat Oncol Biol Phys 63(suppl 1):S412
Feigenberg S, Yu JQM, Eade TN et al (2007) FDG PET response by 3 months following stereotactic body radiotherapy for non-small cell lung cancer may be an early surrogate of local failure. Int J Radiat Oncol Biol Phys 69(3 suppl 1):S479–S480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, S.S., Teh, B.S., Mayr, N.A. et al. Imaging follow-up after stereotactic ablative radiotherapy (SABR) for lung tumors. J Radiat Oncol 1, 11–16 (2012). https://doi.org/10.1007/s13566-012-0014-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-012-0014-2