Abstract
Melanoma brain metastasis poses a difficult therapeutic challenge. Melanoma is perceived as a radioresistant histology and is thought to be less responsive to whole brain radiotherapy (WBRT). Stereotactic radiosurgery (SRS) is regarded as one of the treatment options that may improve local control in patients with a small number of lesions. A total of 28 patients with one to three brain metastases from melanoma primary (43 lesions) were treated with Gamma Knife-based SRS. Eight patients received WBRT. The median marginal dose to the lesions was 20 Gy (range 15–22 Gy) delivered to the 50% isodose line. The cumulative treatment volume ranged from 0.587 to 24.00 ml (median 4.0). Median follow-up for patients was 5.9 months (range 1.3 to 30.9 months). The 3-, 6-, and 12-month overall survival (OS) rates were 78.6%, 52.4%, and 26.4%, respectively, for the whole group. The corresponding freedom from local progression (FFLP) rates were 78.5%, 68.7%, and 61.1%. The corresponding free from distant brain failure (FFDBF) and free from intracranial failure (FFICF) rates were 71.7%, 47.5%, and 36.9% and 68.9%, 51.7%, and 28.2%, respectively. The addition of WBRT did not impact on OS, FFLP, FFDBF, or FFICF. In patients who are reliable for close follow-up with serial MRI of the brain, SRS alone may be presented as a treatment option. The role of WBRT in patients with limited melanoma brain metastases would be best answered in a phase III randomized trial setting.
Similar content being viewed by others
Introduction
Melanoma has a high propensity to metastasize to the brain and represents the third most cause of brain metastasis [1]. A significant proportion of patients with stage IV melanoma will eventually develop brain metastasis. For patients with a single brain metastasis, surgical resection followed by whole brain radiotherapy (WBRT) or WBRT followed by stereotactic radiosurgery (SRS) has been demonstrated to improve intracranial tumor control compared to WBRT alone [2, 3]; for patients with two to three or two to four brain metastases, WBRT followed by SRS has been demonstrated to improve intracranial tumor control compared to WBRT alone [3, 4]. Recent data from phase III trials comparing SRS alone and SRS + WBRT in patients with one to three or one to four brain metastases showed no compromise of survival when WBRT was omitted or delayed despite a higher risk of intracranial failure [5, 6]. Melanoma is perceived as a radioresistant histology, and the value of addition of WBRT to SRS in this setting is highly debated [7]. The aim of this study was to examine the outcomes of patients with one to three melanoma brain metastases treated with SRS with or without WBRT.
Materials and methods
Exempt review was granted by our cancer hospital institutional review board for the collection of data of patients with melanoma brain metastases treated with Gamma Knife (GK)-based SRS in our department. All patients were then deidentified before data analysis. In the period of 2000 to 2007, a total of 28 patients (14 male and 14 female) with one to three brain metastases from melanoma primary (43 lesions) were treated with SRS. The median age was 56.5 years (range 27–81). The median Karnofsky performance status was 90 (range 70–100). There were one lesion in 18 patients, two lesions in 5 patients, and three lesions in 5 patients. The distribution of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) classes was: RPA I—1 patient and RPA II—27 patients. Twenty-six patients received systemic therapy and 4 had control of their extracranial disease. Eight patients received WBRT (30 Gy in ten fractions). The decision to give WBRT prior to SRS was based on individual radiation oncologists’ preference.
Technical details
Leksell 60Co GK (Elekta AB, Stockholm, Sweden) was used to deliver SRS for all patients. B and 4C models were used before and after June 2006, respectively. Lesions not exceeding 4 cm in maximum diameter were eligible. On the day of the Gamma Knife procedure, a Leksell stereotactic head frame was fixed to the patient’s skull with local anesthetic injected into the pin sites. To enable us to visualize small metastatic lesions, a thin-cut (1.2–1.5 mm slices) stereotactic multiplanar, gadolinium (double contrast or Multihance)-enhanced MRI, including a spoiled gradient recalled acquisition (SPGR) sequence was performed for target delineation. Every stereotactic MRI study was jointly reviewed by the treating neurosurgeon and radiation oncologist as well as the reading neuroradiologist to confirm the number of metastasis prior to treatment planning. Treatment planning was performed using the computer planning software provided by Leksell 60Co Gamma Knife (Elekta AB, Stockholm, Sweden).
The prescribed dose was based on the size of the individual lesion. For tumors ≤2, 2.1–3.0, and 3.1–4.0 cm, a single dose of 20–22, 18, and 15 Gy was given, respectively. The median marginal dose to the lesions was 20 Gy (range 15–22 Gy) delivered to the 50% isodose line. The cumulative treatment volume ranged from 0.587 to 24.00 ml (median 4.0).
Follow-up
After Gamma Knife-based SRS, a repeat MRI of the brain was performed every 3–4 months for follow-up. A spoiled gradient recalled acquisition sequence was always included for better delineation of brain metastases. In nearly all cases, patients would be followed by radiation oncology, neurosurgery, medical oncology, and neuro-oncology. Any significant tumor enlargement, as noted in the radiology report or the chart notes, was scored as a local recurrence/progression. Typically, when there was any doubt as to whether the radiographic changes represented local recurrence/progression, the case was discussed in our interdisciplinary neuro-oncology conference and the conclusion was documented in the chart.
End points and statistical analysis
StatView statistical software (SAS Institute, Inc.) was used for analysis. In this study, the end points being examined included overall survival (OS), progression-free survival space (PFS), local control (LC), freedom from local progression (FFLP), freedom from distant brain failure (FFDBF) and freedom from intracranial failure (FFICF). OS was defined as no death from any cause, whereas PFS was defined as survival with no progression anywhere. LC was defined as no local progression for each individual metastasis, and FFLP was defined as no local progression for each individual patient. FFDBF was defined as no out of SRS field failure, and FFICF was defined as no intracranial progression, either in field or out of field, for each individual patient. The above-mentioned end points were calculated from the date of the patient’s SRS procedure, and they were recorded in months. Kaplan–Meier analysis was used to calculate on the above-mentioned end points.
Results
Survival
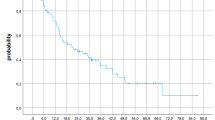
Median follow-up for patients was 5.9 months (range 1.3 to 30.9 months). The 3-, 6-, and 12-month OS rates were 78.6%, 52.4%, and 26.4%, respectively, for the whole group. The corresponding PFS rates were 57.1%, 32.1%, and 20.1%. The addition of WBRT did not impact on OS or PFS (Table 1). Figures 1 and 2 show OS and PFS, respectively, with and without WBRT.
Intracranial tumor control
Nine (32%) patients developed local progression, six in the SRS and three in the WBRT ± SRS groups. The 3-, 6-, and 12-month LC rates were 78.7%, 68.4%, and 63.2%, respectively. The corresponding FFLP rates were 78.5%, 68.7%, and 61.1%. Fourteen patients (50%) developed distant brain failure, 11 in the SRS and 3 in the WBRT ± SRS groups. Twenty patients had intracranial progression, 15 (4 had local progression only, 9 had distant progression only, and 2 had both local and distant progression) in the SRS, and 5 (2 had local progression only, 2 had distant progression only, and 1 had both local and distant progression) in the WBRT ± SRS groups. The corresponding FFDBF and FFICF rates were 71.7%, 47.5%, and 36.9% and 68.9%, 51.7%, and 28.2%, respectively. The addition of WBRT did not impact on LC, FFLP, FFDBF, or FFICF (Table 1). Figures 3, 4, 5, and 6 show LC, FFLP, FFDBF and FFICF, respectively, with and without WBRT.
Salvage therapy
Five patients who had WBRT and SRS received temozolomide for salvage. For the remaining 15 patients who had SRS alone, salvage therapy included WBRT, repeat SRS, or temozolomide.
Toxicities
One of the patients developed symptoms related to subacute radiation-induced edema and treated with steroid therapy with improvement. Another patient developed radiation necrosis 10 months after SRS and was treated with surgery.
Discussion
Traditionally, whole brain radiation therapy has been used for treatment of brain metastases, including those from melanoma, especially in the setting of multiple brain metastases [1]. To improve treatment outcomes, in patients with limited number of brain metastases (one to three lesions), SRS has been used as a boost after WBRT. In the RTOG 9508 trial, 333 patients with one to three brain metastases were randomized into WBRT only and WBRT plus SRS boost arms. There was a survival advantage demonstrated in the SRS boost arms for patients with a single metastasis (median survival interval—6.5 vs. 4.9 months for WBRT alone arm) [3]. Overall, patients who underwent SRS were more likely to have a stable or improved Karnofsky performance status at 6-month follow-up compared to patients who only had WBRT [3]. Patients with RPA class I or more favorable histology had improved survival.
Melanoma is being regarded as a radioresistant histology, and brain metastases from melanoma will theoretically show better response with an SRS boost after WBRT. Controversy exists as to whether WBRT would impact on intracranial tumor control and survival in patients with brain metastases from radioresistant histologies including melanoma [7]. There were three randomized trials comparing SRS alone and WBRT plus SRS [5, 6, 8]. All three studies included brain metastases from various histologies. In the trial from Japan, 132 patients with one to four brain metastases <3 cm in diameter were randomized into WBRT plus SRS and SRS alone arms [6]. There was no difference in the median survival time between the two arms (7.5 vs. 8 months for WBRT + SRS and SRS alone arms). The 12-month brain tumor recurrence rate at 1 year was higher in the SRS alone arm 76.4% vs. 46.8% in the WBRT + SRS arm. Salvage brain treatment was less frequently required in the WBRT + SRS arm compared to the SRS alone arm. However, there was no difference in terms of deaths attributed to brain metastases. Subsequent companion study based on this group of patients showed statistically significant differences in minimental state examination according to total tumor volume, extent of tumor edema, age, and Karnofsky performance status [9]. The time to deterioration of the neurocognitive function was significantly shorter in patients who had SRS alone (7.6 vs. 16.5 months in patients who had WBRT + SRS). The authors concluded that brain tumor control is the most important factor for stabilizing neurocognitive function although the long-term adverse effects of WBRT on neurocognitive function might not be negligible. In the phase III randomized trial from M.D. Anderson Cancer Center, Chang et al. randomized 58 patients with one to three brain metastases into one of the two arms, WBRT plus SRS, and SRS alone. Melanoma was one of the histologies included. Despite the much better intracranial tumor control (73% vs. 27% for patients who had SRS alone), which was statistically significant, patients who underwent WBRT plus SRS had worse overall survival [5]. The investigators observed that patients treated with SRS plus WBRT were at a greater risk of a significant decline in learning and memory function by 4 months compared with the patients treated with SRS alone. The European Organisation for Research and Treatment of Cancer conducted a phase III trial assessing whether adjuvant WBRT increased the duration of functional independence after surgery or SRS for patients with one to three brain metastases including various histologies [8]. Although addition of WBRT reduced intracranial relapse and neurologic deaths, it did not impact on OS or duration of functional independence.
In an Eastern Cooperative Oncology Group (E 6397) phase II trial of SRS alone for patients with one to three radioresistant brain metastases, 36 patients were enrolled and 31 were eligible for analysis [10]. At a median follow-up sign of 32.7 months, the intracranial failure with SRS alone was 25.8% and 48.3% at 3 and 6 months. The in-field failure rates were 19.3% and 32.2% at 3 and 6 months. The corresponding out-of-field failure rates were 16.2% and 32.2%. Given the high rates of in-field and distant brain failure, the investigators cautioned the routine use of SRS for patients with one to three radioresistant brain metastases.
In our study, the addition of WBRT did not impact on survival outcomes nor intracranial tumor control. The main advantage of the addition of WBRT to SRS for patients with one to three brain metastases is to potentially improve local control, distant brain control, and overall survival. This is under the assumption that intracranial tumor progression will invariably lead to decline of neurocognitive function and decreased survival. So far, there has only been three fully reported phase III randomized trials comparing WBRT plus SRS and SRS alone for patients with limited number of brain metastases, including various histologies [5, 6, 8]. Those three trials showed conflicting results regarding the relative contributions of intracranial tumor progression and in the decline of neurocognitive function. Various retrospective SRS series of patients with radioresistant or melanoma brain metastases did not consistently show the benefit of addition of WBRT in patients receiving SRS [7]. At our institution, patients with one to three radioresistant brain metastases are typically treated with SRS alone followed by close observation. Stereotactic MRI on the day of the procedure is done using double contrast or Multihance, and SPGR sequence with 1.2–1.5-mm slices is always included. Very small metastatic lesions are more readily identified. Serial MRI of the brain is typically performed at least at 3 months, earlier if they are new or worsening neurologic symptoms. Using this approach, we are able to detect new brain metastases when they are still very small and asymptomatic. This is most likely the reason why no differences were appreciated in terms of overall survival in the three randomized trials and in our study.
There are certainly limitations of this current study, and its results are to be interpreted with caution. This study is a retrospective review, and it is subject to bias. The patient number was relatively small and unbalanced, and it was not powered enough to detect any difference between the two groups. Furthermore, the follow-up times were relatively short. Furthermore, formal neurocognitive testing was not performed in patients in this retrospective study. To better define the role of WBRT in the management of patients with limited number (one to three or one to four) brain metastases from radioresistant primaries, a phase 3 randomized trial would be necessary. In patients who are reliable for close follow-up with serial MRI of the brain, SRS alone may be a reasonable treatment option.
Conclusions
Based on this small retrospective study, SRS could yield fair LC and FFLP in patients with one to three brain metastases from melanoma. However, there was a high incidence of distant brain failure (50%) and OS remained poor (26.4% at 12 months) regardless of whether WBRT was offered or not. The addition of WBRT did not significantly impact any of the endpoints examined. The role of WBRT in patients with limited melanoma brain metastases would be best answered in a phase III randomized trial setting. In patients who are reliable for close follow-up with serial MRI of the brain, SRS alone may be presented as a treatment option.
References
Sloan AE, Nock CJ, Einstein DB (2009) Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 16(3):248–255
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(8):494–500
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363(9422):1665–1672
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45(2):427–434
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295(21):2483–2491
Lo SS, Chang EL, Suh JH (2005) Stereotactic radiosurgery with and without whole-brain radiotherapy for newly diagnosed brain metastases. Expert Rev Neurother 5(4):487–495
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141. doi:10.1200/JCO.2010.30.1655
Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68(5):1388–1395
Manon R, O’Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M (2005) Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 23(34):8870–8876
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, S.S., Radawski, J.D., Nelson, A. et al. Stereotactic radiosurgery with or without whole brain radiotherapy for patients with one to three melanoma brain metastases. J Radiat Oncol 1, 73–79 (2012). https://doi.org/10.1007/s13566-012-0006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-012-0006-2