Abstract
Mannose specific jacalin-related lectins or agglutinins (mJRLs) constitute an important superfamily of proteins known to play vital roles in various biological processes. In the present study, a cDNA having 876 bp open reading frame (ORF) coding for mJRL of 291 amino acids residues was cloned from pinna of Cycas annaikalensis which is endemic to Western Ghats, India and designated as C. annaikalensis pinna lectin (CAPL). Expression of the coding sequence under the control of a T7 promoter in E. coli produced 31 kDa protein. The purified recombinant protein had shown agglutination with erythrocytes of rabbit blood. The deduced amino acid sequence of CAPL showed two sugar binding sites (also determined to be jacalin-like lectin domains) and 95% similarity with C. revoluta leaf lectin (CRLL) protein. Further, a monomeric protein of CAPL consisting of mannose binding residues and jacalin motifs that are having 35–90% similarities with mJRLs which have already been reported. A phylogenetic tree exhibited the grouping of CAPL into a subclade different from that of the CRLL. Also, a model of cycas leaf lectin was built by homology modeling using 1ZGRA (Parkia platycephala seed lectin) as a template for the construction of three-dimensional structures. Structural modeling and docking studies were completed using Discovery studio version 2.1. This study, first of its kind, reports mJRLs from the Indian gymnosperm.





Similar content being viewed by others
Abbreviations
- mJRLs:
-
Mannose binding jacalin related lectins
- CAPL:
-
Cycas annaikalensis pinna lectin
- CRLL:
-
Cycas revoluta leaf lectin
- gJRLs:
-
Galactose binding JRLs
- SOPMA:
-
Self-optimized prediction method in alignment
- RMSD:
-
Root mean square deviation
References
Astoul HC, Peumans WJ, Van Damme EJM, Barre A, Bourne Y, Rouge P (2002) The size, shape and specificity of the sugar-binding site of the jacalin-related lectins is profoundly affected by the proteolytic cleavage of the subunits. Biochem J 367:817–824
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bichem 72:248–254
Das B, Mahender G, Koteswara Rao Y, Thirupathi P (2006) A new biflavonoid from Cycas beddomei. Indian J Chem Sect B Org Chem Incl Med Chem 45B:1933–1935
Gallego De Sol F, Nagano C, Cavada BS, Calvete JJ (2005) The first crystals structure of Mimosoidea lectin reveals a novel quaternary arrangement of a widespread domain. J Mol Biol 353(3):574–583
Gasteiger E, Hoogland C, Gattiker A., Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: The proteomics protocols handbook, pp 571–607. Humana press
Geourjon C, Deleage G (1995) SOPMA: significant improvement in protein secondary structure prediction by consensus prediction from multiple alignments. Cabios 11:681–684
Haraguchi T, Nomura K, Yagi F (2006) Cloning and expression of a mannose-binding jacalin-related lectin from leaves of Japanese cycad (Cycas revoluta Thunb.). Biosci Biotechnol Biochem 70:2222–2229
Jiang SY, Ma Z, Ramachandran S (2010) Evolutionary history and stress regulation of the lectin super family in higher plants. BMC Evol Biol 10(79):1–24
Kai G, Zhao L, Zheng J, Zhang L, Miao Z, Sun X, Tang K (2004) Isolation and characterization of a new mannose-binding lectin gene from Taxus media. J Biosci 29(4):399–407
Kumar S (2015) Biopesticide: an environment friendly pest management strategy. J Biofertil Biopestici 6(1):127
Lannoo N, Van Damme EJM (2014) Lectin domains at the frontiers of plant defense. Front Plant Sci 5(397):1–16. https://doi.org/10.3389/fpls.2014.00397
López S, Armand-Úgon M, Bastida J, Viladomat F, Esté JA, Stewart D, Codina C (2003) Anti-human immunodeficiency virus type 1 (HIV-1) activity of lectins from Narcissus species. Planta Med 69:109–112
Ma QH, Tian B, Li YL (2010) Over expression of wheat jasmonate regulated lectin increases pathogen resistance. Biochimie 92(2):187–193
Ma QH, Zhen WB, Liu YC (2013) Jacalin domain in wheat Jasmonate regulated protein TA-JA1 confers agglutinating activity and pathogen resistance. Biochemie 95(2):359–365
Nakamura-Tsuruta S, Uchiyama N, Peumans WJ, Van Damme EJM, Totani K, Ito Y, Hirabayashi J (2008) Analysis of the sugar-binding specificity of mannose-binding-type Jacalin-related lectins by frontal affinity chromatography—an approach to functional classification. The FEBS journal 275(6):1227–1239
Peumans WJ, Barre A, Bras J, Rougé P, Proost P, Van Damme EJM (2002) The liverwort contains a lectin that is structurally and evolutionary related to the monocot mannose-binding lectins. Plant Physiol 129(3):1054–1065
Radha P, Singh R (2008) Ethnobotany and conservation status of Indian Cycas species. Encephalartos 93(1):15–21
Raval S, Gowda SB, Singh DD, Chandra NS (2004) A data base analysis of jacalin like lectin sequences-structure-function relationships. Glycobiology 14(2):1247–1263
Rüdiger H, Gabius HJ (2001) Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj J 18(8):589–613
Sharma HC (2008) Biotechnological approaches for pest management and ecological sustainability. CRC Press, Boca Raton
Sharma S, Gokhale SM (2014) Enzymatic cleavage of cell surface protein of pig and cow erythrocytes and its effect on concanavalin-mediated agglutinability. Indian J Bichem Biophys 51:378–387
Sharma A, Vijayan M (2011) Quaternary association in β-prism I fold plant lectins; insights from X-ray crystallography, modellingand molecular dynamics. J Biosci 36(5):793–808
Sharma A, Chandran D, Singh DD, Vijayan M (2007) Multiplicity of carbohyderate-binding sites in β-prism fold lectins; occurrence and possible evolutionary implications. J Biosci 32(2):1089–1110
Shimokawa M, Haraguchi T, Minami Y, Yagi F, Hiemori K, Tateno H, Hirabayashi J (2016) Two carbohydrate recognizing domain from Cycas revoluta leaf lectin show the distinct sugar binding specificity—a unique manno oligosaccharide recognition by N-temrinal domain. J Biochem 106(1):27–32
Singh R, Radha P, Khuraijam JS (2015) A new species, a new combination and a new subsection of Cycas from Odisha, Northern Eastern Ghats of India. Asian J Conserv Biol 4(1):3–14
Subramanyam S, Smith DF, Clemens JC, Webb MA, Sardesai N, Willian CE (2008) Functional characterisation of HFRI, a high mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol 147(3):1412–1426
Tateno H, Winter HC, Petryniak J, Goldstein IJ (2003) Purification, characterization, molecular cloning, and expression of novel members of jacalin-related lectins from rhizomes of the true fern Phlebodium aureum (L) J. Smith (Polypodiaceae). J Biol Chem 278(13):10891–10899
Teixeira EH, Arruda FVS, Nascimento KS, Carneiro VA, Nagano CS, Silva BR, Sampaio AH, Cavada BS (2012) Biological. Applications of plants and algae lectins an overview. In: Chang CF (ed) Carbohydrates—comprehensive studies on glycobiology and glycotechnology. Intech Rijeka, pp 553–558
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W improving the sensitivity of progressive multiple alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acid Res 22(22):4673–4680
Van Damme EJ, Peumans WJ, Bare A, Rouge P (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological role. Crit Rev Plant Sci 17(6):645–662
Vandenborre G, Van Damme EJM, Smagghe G (2009) Natural products: plant lectins as important tools in controlling pest insects. In: Ishaaya I, Horowitz AR (eds) Biorational control of arthropod pests: application and resistance management. Springer, Dordrecht, pp 163–187
Vandenborre G, Smagghe G, Van Damme EJM (2011) Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72:1538–1550
Xiang Y, Song M, Wei Z, Tong J, Zhang L, Xiao L, Ma Z, Wang Y (2011) A jacalin-related lectin-like gene in wheat is a component of the plant defence system. J Exp Bot 62(15):5471–5483
Yagi F, Iwaya T, Haraguchi T, Goldstein IJ (2002) The lectin from leaves of Japanese cycad, Cycas revoluta Thunb. (Gymnosperms), is a member of jacalin-related family. Eur J Biochem 269(17):4335–4341
Yong WD, Xu YY, Xu WZ, Wang X, Li N, Wu JS, Liang TB, Chong K, Xu ZH, Tan KH, Zhu ZQ (2003) Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2. Planta 217(2):261–270
Acknowledgements
Author Dr. P. Radha thanks University Grant Commission (UGC), Govt. of India for financial support through UGC-PDF for women scheme (PDFWM-2011-12-GE-TAM-9600(SA-II). Prof. V. D. Reddy and Prof. K.V. Rao, Centre for Plant Molecular Biology (CPMB), Osmania University, Hyderabad and Dr. M. Shesheer Kumar, VSS Pavan Kumar and M. Ravi Chintada, RAS life sciences Pvt Ltd, Hyderabad, India are acknowledged for their constant support and suggestion during this study.
Author information
Authors and Affiliations
Contributions
RU designed and conducted the gene isolation; cloning and biochemical characterization studies. PR created the whole idea, experiments and performed cloning, bioinformatics analysis. RU, PR drafted the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
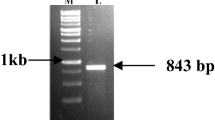
Amplification of C. annaikalensis pinnae lectin gene (CAPL): M. Gene Ruler 1 kbDNA ladder; 1, 2 CAPL amplified product at ~ 876 bp (TIFF 40927 kb)
Supplementary Fig. 2
Restriction digestion analysis of pTNOT-CAPL: M. GeneRuler 1 kb DNA ladder; 1, 2, and 3. release of ~ 900 bp band corresponding to CAPL gene and vector backbone at 3 kbp up on digestion with Bam HI and Not I (TIFF 36268 kb)
Supplementary Fig. 3
Restriction analysis of pET28a+ CAPL: M. GeneRuler DNA ladder mix; 1. Undigested pET28a+ CAPL plasmid; 2. 2, 3, 4 release of ~ 900 bp band corresponding to CAPL gene up on digestion with Bam HI and Not I (TIFF 33317 kb)
Supplementary Fig. 4
The full-length cDNA sequence and deduced amino acid sequence of CAPL. The start codon (ATG) is in italics and the stop codon (TGA) is in italics and underlined. The underlined sequence is the sugar binding sites; the bold residues indicate the potential glycosylation sites; the shades residues are GXXXD motifs (TIFF 31839 kb)
Supplementary Fig. 5
Secondary structure analysis of CAPL using SOPMA secondary structure prediction method: CAPL 291 amino acid contribution in the form of α helices (54 aa), beta turns (27 aa), random coil (124 aa) and extended strand (86 aa) were represented by blue, purple, green and red lines respectively (TIFF 5108 kb)
Supplementary Fig. 6
Phylogenetic analysis of CAPL with other mJRLs: Heltuba-Helianthus tuberosus lectin, Calsepa-Calystegia sepium lectin;CCA-Castanea crenata; MornigaM-Morus nigra; PK-Parkia platycephala lectin; Banlec-Musa acuminate; KM + -Artocarpus integrifolia lectin; PAL-Phlebodium aureum lectin; Orysata-Oryza sativa lectin; CRLL-Cycas revoluta leaf lectin; C-a. CAPL; C.c.-CCPL; C-ru-C. rumphii lectin (TIFF 3511 kb)
Supplementary Fig. 7
Ramachandran plot analysis of modelled structure of CAPL protein showed 90.1% residues are favored regions, whereas 7.2% and 2.7% of the protein in allowed regions and in the outlier region respectively (TIFF 26621 kb)
Supplementary Fig. 8
Three dimensional structure of CAPL with their sugar binding residues (amino acids 139-143 and 277-281) (TIFF 32791 kb)
Rights and permissions
About this article
Cite this article
Radha, P., Urla, R. Cloning and characterization of mannose binding jacalin related lectin encoding gene from Indian Cycas (Cycas annaikalensis Singh and Radha). J. Plant Biochem. Biotechnol. 29, 314–322 (2020). https://doi.org/10.1007/s13562-019-00513-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-019-00513-3