Abstract
Systemic and localized scleroderma are difficult to manage diseases with no accepted gold standard of therapy to date. Phototherapeutic modalities for scleroderma show promise. A PubMed search of information on phototherapy for scleroderma was conducted. The information was classified into effects on pathogenesis and clinical outcomes. Studies on photopheresis were excluded. There were no randomized, double-blind, placebo-controlled studies, and only three controlled studies. The vast majority of identified studies evaluated ultraviolet A1 (UVA1) phototherapy. More rigorous studies are needed to evaluate phototherapy in the treatment of scleroderma. Based on the limited studies available, 20–50 J/cm2 of UVA1 therapy 3–4 times a week for 30 treatments is recommended.
Similar content being viewed by others
Introduction: Background on Morphea/Scleroderma
Scleroderma is a chronic autoimmune disease associated with cutaneous, joint, and internal organ involvement. Cutaneous scleroderma is characterized by enhanced fibroblast activity leading to hypertrophic dermal collagen. There are localized and systemic forms of scleroderma. The localized forms include morphea and linear scleroderma. Localized scleroderma has a better prognosis and does not involve internal organs. There are currently no curative treatments for scleroderma. Current treatments include immunosuppressants; intralesional, topical, and oral steroids; topical vitamin D; and phototherapy. This review serves to provide insight into the use of phototherapy in the management of scleroderma. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Phototherapy in Dermatology
Phototherapy modalities utilize specific wavelengths of the electromagnetic spectrum to disrupt the dysfunctional and pathologic tissue that has developed in some patients with skin disease. Various phototherapy modalities possess anti-inflammatory effects [1]. The longer the wavelength of phototherapy, the deeper in the dermis it penetrates [2]. Current phototherapeutic modalities being used for dermatoses include broadband ultraviolet B (UVB 290–320 nm), narrowband UVB (311–313 nm), excimer laser (308 nm), ultraviolet A (UVA 320–400 nm), ultraviolet A1 (UVA1 340–400 nm), psoralen and UVA (PUVA), and extracorporeal photochemotherapy.
Mechanism Behind Phototherapeutic Modalities Used in Scleroderma
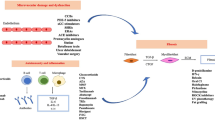
A common theory behind the mechanism of phototherapy in scleroderma is that light is converted to chemical energy resulting in the increase of reactive oxygen species or singlet oxygen production, which can modulate the expression of cytokines [3, 4]. Ultraviolet radiation includes UVA and UVB therapy, with UVA1 studied the most. UVA1 can have an output categorized as low (10–30 J/cm2), moderate (40–70 J/cm2), or high (up to 130 J/cm2).
UVA1 radiation increases collagenase [also known as the matrix metalloproteinase-1 (MMP-1)] gene, mRNA, and protein expression by fibroblasts [5–9]. In mice models, UVA1 radiation reduces fibroblast proliferation in a dose-dependent fashion [10, 11]. Additionally, UVA1 radiation administered three times a week showed decreased hydroxyproline and collagen levels in a dose-dependent fashion [11]. The quality of the collagen is altered after UVA1 therapy, as collagen appears less dense and smoother compared to before treatment [12]. Decorin (a proteoglycan component of connective tissue) mRNA levels are lower in lesional scleroderma versus non-lesional skin, and decorin levels are increased after UVA1 phototherapy [13]. Transforming growth factor beta (TGF-β) protein levels (TGF-β is profibrotic) are inversely correlated with decorin levels. On the other hand, another study showed that after UVA1 phototherapy, decorin was decreased in the upper to middle dermis, although decorin slightly increased in the papillary dermis [14]. In patients, UVA has been shown to reduce collagen I, collagen III, and TGF-β and increase interferon-γ [9]. UVB radiation increases alpha melanocyte-stimulating hormone (α-MSH) receptor synthesis in keratinocytes and melanocytes [15]. Human fibroblast dermal cultures treated with α-MSH demonstrated an increase in MMP-1 mRNA, indicating that α-MSH may be one of UVB's mediators of anti-fibrosis [16].
The source of the mediators that contribute to the reduction in sclerosis comes mostly from the dermis. Subsequently, certain parts of the dermis may be impacted more than others. An image analyzer showed a greater reduction in collagen fibers in the upper and middle dermis and less reduction in the lower dermis [12]. In 18 patients treated with UVA1, the MMP-1 level was higher in the papillary layers and lower in the reticular layers [17]. The anti-fibrotic effects of phototherapy may not come exclusively from the dermis. Samples taken 18 h after the final UVA1 treatment in a set of patients showed an increase in interstitial collagenase in the upper layer of keratinocytes, melanocytes, and endothelial cells [5].
Evidence supports the regimen of multiple UVA1 therapy sessions a week. The anti-sclerotic effects of a single exposure of UVA1 effects are typically seen to last less than 1 week. In human skin, mRNAs of type I and III procollagen were decreased and MMP-3 was increased after 3 days of a single UVA1 dose [18]. MMP-1 and MMP-3 were upregulated for 3 to 5 days, while procollagen levels were suppressed for at least 7 days [18]. In this small study, anti-fibrotic responses became refractory to multiple UVA1 exposures over the course of 1 week, as repeated exposures weekly showed no reduction in type I procollagen levels [18].
UVA1 therapy can have an immunomodulatory effect on lesional skin. UVA1 can reduce inflammation in the dermis [12]. UVA1 causes apoptosis of T-cells [19]. Patients with morphea exposed to UVA1 with a dose of 30 J/cm2 and a cumulative dose of 900 J/cm2 were found to have an increase in CD34+ dendritic cells [20]. Human beta defensin[s] (HBD), interleukin (IL)-6 and IL-8 are downregulated in patients with localized scleroderma treated with UVA1 phototherapy [6]. On the other hand, another study showed that UVA1 induces MMP-1 through a mechanism involving IL-1 and IL-6 [21].
UVA1 radiation may induce oxidative stress, as evidenced by an increase in UVA1-induced heme oxygenase-1 in fibroblasts [7]. Glutathione was lower in systemic sclerosis (SSc) fibroblasts than control samples, but glutathione was increased and became equivalent between normal and SSc fibroblasts after in vitro irradiation with UVA1 [8]. Thus, the SSc fibroblasts may be more susceptible to phototherapy-induced oxidative stress than normal fibroblasts [8]. Additionally, heme oxygenase-1 may reduce fibrotic conditions via TGF-β [22]. UVA1 may play a role in angiogenesis. In patients exposed to UVA1 phototherapy for 14 weeks, there was an increase in CD34+ cells and an increase in vascular endothelial growth factor (VEGF) [23]. The neuroendocrine system may be involved, as UVA1 therapy decreases dermal expression of neuron-specific enolase, which correlated with softening of skin lesions in patients with SSc with acral lesions [24].
UVB phototherapy results in DNA damage, forming cyclobutane pyrimidine dimers between nucleotides [25]. There is evidence that broadband UVB can induce interstitial collagenase, stromelysin, and IL-6 [26]. There may be an interplay between these enzymes and cytokines [26]. Broadband UVB radiation can induce production of MMP-1 in fibroblasts [27]. When keratinocytes are exposed to UVB, there is an increase in IL-1α and IL-6, which induced MMP-1 [27]. Human keratinocytes cultured in a model system exposed to 300 J/cm2 of broadband UVB produced IL-1α, IL-6, and tumor necrosis factor alpha (TNF-α) [28].
PUVA is another modality that can be used for scleroderma. PUVA can lead to apoptosis of T-cells in the dermis [19]. In patients with SSc treated with PUVA, the majority of patients experienced an increase in circulating TNF-α levels, E-selectin, and vascular cell adhesion molecule (VCAM). In the majority of patients, there was a reduction in VEGF and TGF-β [29]. On the other hand, in patients with morphea treated with PUVA, there was a fall in serum VCAM molecules and an increase in TNF-α in most patients [30]. In a bleomycin-induced scleroderma rat model, PUVA treatment reduced dermal thickness and hydroxyproline content and downregulated expression of type I and III collagen genes [10]. In one patient with SSc, treatment with oral PUVA therapy three times a week for 4 weeks resulted in loosening of collagen, reduction in edema, and decreased CD34+ cells [31]. Bath PUVA treatment has effects on collagen cross-links in human skin samples of scleroderma, reducing hydroxylysylpyridinoline and lysylpyridinoline [32]. UVA1 treatment affected collagen fibrils mostly in the upper reticular dermis [33], whereas PUVA affected collagen fibrils in the upper and middle reticular layers [33]. Additionally, collagen fibrils decreased and new fibrils developed, suggesting UVA1 and PUVA phototherapies’ impact on sclerotic lesions occurs via collagen degradation and new collagen synthesis [33].
Other modalities have also been studied. Photodynamic therapy (PDT) with 5-aminolevulinic acid (5-ALA) treatment of scleroderma fibroblasts increased MMP-1 and MMP-3, and there was a decrease in collagen type 1 mRNA as early as 6 h after treatment [34]. Keratinocytes exposed to PDT with 5-ALA had an increase in IL-1α and TNF-α [35]. In fibroblasts that were incubated with keratinocytes pre-exposed to PDT with 5-ALA, there was an increase in MMP-1 and MMP-3; Karrer et al. [35] subsequently suggested paracrine signaling between the phototherapy exposed keratinocytes and the fibroblasts. Furthermore, an IL-1 antagonist reversed the induction of MMP-1 and MMP-3 in fibroblasts [35]. Blue light up to 453 nm is toxic to cultured T cells, causing apoptosis, but was nontoxic for other skin cell types [36].
The Use of Phototherapy in Dermatology
Phototherapy is commonly used for many dermatoses, but there is less usage for scleroderma. Of 653 patients using phototherapy in a Brazilian clinic, 11 were there for scleroderma treatment [37]. In a multi-center response from 155 British pediatric physicians, PUVA was the most popular phototherapy modality (38%), followed by narrowband UVB (23%) and UVA1 (16%) for morphea [38]. These same clinicians were also asked what would be the best treatment option overall in their opinion for active morphea: 17% responded phototherapy and about 2/3 of these responses were for UVA1, which was only accessible to 27% of respondents [38]. Phototherapy for adult skin disorders is almost exclusively provided by dermatologists [39]. In a survey of physicians treating juvenile localized scleroderma in the UK, 19 of 28 pediatric dermatologists used UV therapy, whereas 0 of 10 pediatric rheumatologists used UV therapy [40]. A self-reported survey of dermatologists and rheumatologists revealed that 20% of dermatologists (n = 40) and 10.6% of pediatric dermatologist (n = 47) used phototherapy [41].
Clinical Evidence of Phototherapy’s Efficacy
Search Method
A PubMed search was performed with the Boolean search terms ‘scleroderma’ OR ‘morphea’ OR ‘crest’ AND ‘phototherapy.’ The search years yielded were from 1978 to 2016. Clinical articles in a non-English language were excluded.
UVA1
UV therapy for patients with localized scleroderma was introduced as PUVA in 1994 [42]. In 1995, Kerscher et al. [43] reported that low-dose UVA1 phototherapy could be used in linear scleroderma. It is unclear whether there is an association between initial skin disease duration and response to UVA1 therapy. A study of ten patients with sclerodermic lesions determined that there was no correlation between disease duration and clinical response with UVA1 [44].
Table 1 lists the clinical reports of UVA1′s efficacy in scleroderma or morphea. It is important to note that covered sclerotic lesions show less improvement after UVA1 therapy [45]. Ultrasound is an objective measure used to assess skin thickness in several UVA studies. Fourteen patients with localized scleroderma treated with UVA1 were evaluated with a 13-MHz ultrasound, and dermal thickness was increased before therapy and decreased from 3.11 ± 1.54 to 2.26 ± 0.86 [46]. Other studies have also supported a correlation of a decrease in dermal thickness when treating with UVA1 therapy [47].
Skin darkness or darkening likely has no effect on UVA1’s efficacy. Forty-seven patients with morphea and 35 with SSc treated with UVA1 phototherapy were analyzed to see whether Fitzpatrick skin type makes an impact on the outcome, with the result being that medium- to high-dose UVA1 had similar efficacy in skin types I–V [48]. There was also no correlation noted for Fitzpatrick skin type and cumulative dose or clinical improvement.
The current evidence suggests that UVA1 effects are dose-related. In an observational report for patients with SSc who completed at least ten treatments, 20% of those treated with low-dose (20–40 J/cm2) UVA1 (n = 5), 83.3% of those treated with medium-dose (>40–80 J/cm2) UVA1 (n = 6), and 100% of those treated with high-dose (>80–120 J/cm2) UVA1 (n = 5) reported improvement [49]. A 14-patient study showed a 70-J/cm2 dose was more effective in treating localized scleroderma lesions than a 20 J/cm2 dose [45]. In six patients with localized scleroderma treated two to three times a week, three patients experienced complete remission [50]. Two of the three received high-dose 100 J UVA1 therapy, of which one of them received 67 treatments and relapsed after 6 months, compared to one patient which received low-dose UVA1 twice weekly for 6 weeks for a total of 39 irradiations and did not relapse after 84-month follow up [50]. A broadband UVA trial examined 63 patients with morphea and 15 patients treated with UVA1 5, 10, or 20 J/cm2 with cumulative doses of 100, 200, and 400 J/cm2, respectively [51]. Clinical improvement was observed in all patients, but there was no comparable difference between the UVA doses.
Long-term outcome of UVA1 therapy is unclear. In a cohort study of 37 patients with morphea with positive clinical benefits from UVA1 treatment 44.5% recurred at 2 years, and 48.4% recurred at 3 years [52]. There was no difference between medium- (60–90 J/cm2) and high-dose (>90 J/cm2) UVA1 phototherapy with respect to recurrence. There was a 1.15-times higher chance of disease recurrence for an increment of 1 year in duration of morphea prior to UVA1 treatment [52].
Broadband UVA
Twelve patients with morphea were treated with low-dose (20 J/cm2) broadband UVA 3 times a week for a total of 20 sessions [12]. Improved softness of skin lesions assessed by palpation was reported as early as three treatments and as late as ten treatments. Longer standing lesions did not respond as well as therapy. As a control, some lesions in the same patients were covered to prevent UVA1 exposure during treatment, and less softening was reported in these covered lesions. After a 1-year follow-up, only two patients reported a reappearance of lesions. Lesions on skin creases or over joints did not respond as well to therapy [12].
PUVA
A 15-year-old male with scleroderma with indurated patches on the trunk and joint restrictions was recalcitrant to hydroxychloroquine, prednisolone, and methotrexate [53]. PUVA at a dose of 0.6 mg/kg twice weekly was subsequently added for 20 sessions over 10 weeks at a cumulative dose of 25.4 J/cm2. Methotrexate was subsequently administered for 7 months. After this period, he was able to make a full fist and increase to a normal range of motion in the ankles; his skin was less indurated and has maintained clinical stability for 2 years [53]. Table 1 lists additional PUVA treatment studies in scleroderma/morphea. PUVA’s effects may be due to local effects rather than systemic effects, as Kerscher et al. [54] noted that residual sclerotic lesions remained in patients in areas hidden from UVA exposure such as parts of the elbow in patients undergoing PUVA.
UVB
A 43-year-old female with radiation-induced morphea was given acitretin daily and UVB three times a week [55]. Two months afterwards there was less induration of her plaque, decreased tenderness, and improved range of motion of the left arm [55]. Eleven patients that underwent phototherapy treatment (seven treated with PUVA and four treated with narrowband UVB) for an average of ten sessions experienced a 48% improvement of their localized scleroderma as indicated by a clinical pinching test [56]. Additionally, the ultrasound examination showed a dermal thickness reduction ranging from 20% to 100% [56]. There was no correlation between the type of phototherapy and clinical response rate [56]. Additional studies on UVB therapies are included in Table 1.
Targeted Phototherapy
Targeted phototherapy is a modality that spares non-lesional skin and is able to deliver a higher fluence. A patient with limited scleroderma and elbow mobility restrictions was treated 2–3 times a week for 13 weeks with 940-nm low-level light therapy with millisecond pulsing and continuous wave modes. Using a sequential pulsing dose on one elbow and continuous wave mode on the other, better results were seen with the pulsing mode showing improvement in skin thickness [57].
Five patients with a total of 11 plaques were treated with a 308-nm monochromatic excimer laser for 4 weeks at a power density of 48 mW/cm2 with a maximum irradiation area of 512 cm2 [58]. The mean number of treatments was seven, and the dose per session was 1.5 J/cm2. The mean total dose was 10 J/cm2. After 4 weeks, 3 out of 5 patients experienced marked improvement with residual hyperpigmentation [58].
A 27-year-old Hispanic female had a contracture of her knee with sclerotic bands on her left lower leg, ankle, and foot that were recalcitrant to methotrexate, UVA1, topical calcipotriene, intralesional triamcinolone acetonide, and physical therapy [59]. The patient was treated with a single treatment of 10.6 µm carbon dioxide laser with a 50 J/cm2 pulse energy, while remaining on methotrexate and topical agents [59]. After 1 week, she experienced an increase in range of motion. After 4 months of follow-up, there was softening of her contracture, and she regained full plantar flexion of her left foot. After a 1-year follow-up, she maintained a full range of motion [59].
Four patients with microstomia and SSc were treated with intense pulsed light. 530–570 mm, 11–14 J/cm2; 10–14 pulse durations was used for the patients every 4 weeks [60]. Patients were followed for 4 months. Three patients experienced an increased interincisal distance of ~1 mm per treatment [60]. One patient did not have improved interincisal distance, but did note activities of daily living became easier. One patient did report recurrence of the stiffness after 3 weeks [60]. Table 1 lists additional reports of targeted phototherapy.
Photodynamic Therapy
In six patients, 20% 5-ALA was applied under occlusion to areas of morphea for 5 h. A band width of 570–670 nm, peak 635-nm light was given. A dose of 25 J/cm2 was given for a total of six weekly treatments. In four of the patients there was clinical improvement as determined by skin scoring, although only one of these patients showed histologic evidence of improvement. The side effects patients reported included burning sensation, dryness, erythema, pigmentation, and pruritus [61]. Table 1 lists an additional study.
Discussion
There are mostly care reports of UVA1, UVB, PUVA (bath and topical), and targeted phototherapies in cases of scleroderma. UVA1 appears to be the most efficacious, but it is also the most studied. There are not many studies on high-dose UVA1, and this needs to be investigated further to assess the optimal dose of UVA to use in scleroderma. Additionally, longer term studies are needed to study the long-term outcome and safety of these treatments. A similar literature review study delineated UVA and PUVA’s efficacy and safety in the context of SSc, localized scleroderma, extragenital lichen sclerosus et atrophicus, sclerodermoid graft-versus-host disease, lupus erythematosus, and other rare sclerotic diseases [62]. This review also asserts that there need to be more rigorous studies to help establish a guide for UVA’s indications as well as its efficacy compared to other conventional medical therapies [62].
Based on the studies available, a reasonable regimen is UVA1 therapy 20–50 J/cm2 3–4 times a week for a total of 30 treatments. There were no double-blind, placebo-controlled trials available, and only three controlled trials. Adverse effects thus far do not correlate with the intensity of therapy. The side effects noted in scleroderma phototherapy include fatigue, a burning sensation, hyperpigmentation, pruritus, erythema, edema, headaches, gastrointestinal upset, and joint and muscle pain. Additionally, one patient undergoing UVA1 phototherapy for disseminated morphea developed bullous pemphigoid after 29 treatments [63]. The long-term effects of UVA1 on patients have not reported skin cancer [64]. Phototherapy should be safe in pregnancy [65] although folate may need to be supplemented as reports show that UVB and solar UV radiation may cause photodegradation [66, 67]. Multiple treatments, as well as limited availability of in-office phototherapy, are barriers to treatment. In a review by Bielsa Marsol [68], it was pointed out that most of the studies for UVA1 therapy were performed in countries where patients are predominantly Fitzpatrick types I–III, although, as noted earlier, the Fitzpatrick skin type thus far has not been shown to have an impact on therapy. Phototherapy may not be as useful for sclerotic diseases that affect structures deeper than the dermis.
References
Morgan MC, Rashid RM. The effect of phototherapy on neutrophils. Int Immunopharmacol. 2009;9(4):383–8.
Kerr HA, Lim HW. Photobiology and phototherapeutics. Adv Dermatol. 2003;19:11–36.
Wlaschek M, Briviba K, Stricklin GP, Sies H, Scharffetter-Kochanek K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J Invest Dermatol. 1995;104(2):194–8.
Amat A, Rigau J, Waynant RW, Ilev IK, Tomas J, Anders JJ. Modification of the intrinsic fluorescence and the biochemical behavior of ATP after irradiation with visible and near-infrared laser light. J Photochem Photobiol B. 2005;81(1):26–32.
Gruss C, Reed JA, Altmeyer P, McNutt NS, Kerscher M. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet. 1997;350(9087):1295–6.
Kreuter A, Hyun J, Skrygan M, Sommer A, Bastian A, Altmeyer P, Gambichler T. Ultraviolet Al-induced downregulation of human beta-defensins and interleukin-6 and interleukin-8 correlates with clinical improvement in localized scleroderma. Br J Dermatol. 2006;155(3):600–7.
Nisar MF, Parsons KS, Bian CX, Zhong JL. UVA irradiation induced heme oxygenase-1: a novel phototherapy for morphea. Photochem Photobiol. 2015;91(1):210–20.
Yin L, Yamauchi R, Tsuji T, Krutmann J, Morita A. The expression of matrix metalloproteinase-1 mRNA induced by ultraviolet A1 (340–400 nm) is phototherapy relevant to the glutathione (GSH) content in skin fibroblasts of systemic sclerosis. J Dermatol. 2003;30(3):173–80.
El-Mofty M, Mostafa W, Esmat S, Youssef R, Bousseila M, Nagi N, Shaker O, Abouzeid A. Suggested mechanisms of action of UVA phototherapy in morphea: a molecular study. Photodermatol Photoimmunol Photomed. 2004;20(2):93–100.
Hayashi S, Ikeda M, Kitamura Y, Hamasaki Y, Hatamochi A. UVA irradiation following treatment with topical 8-methoxypsoralen improves bleomycin-induced scleroderma in a mouse model, by reducing the collagen content and collagen gene expression levels in the skin. J Dermatol Sci. 2012;67(1):20–5.
Ju M, Chen K, Chang B, Gu H. UVA1 irradiation inhibits fibroblast proliferation and alleviates pathological changes of scleroderma in a mouse model. J Biomed Res. 2012;26(2):135–42.
El-Mofty M, Zaher H, Bosseila M, Yousef R, Saad B. Low-dose broad-band UVA in morphea using a new method for evaluation. Photodermatol Photoimmunol Photomed. 2000;16(2):43–9.
Gambichler T, Skrygan M, Tomi NS, Altmeyer P, Kreuter A. Differential expression of decorin in localized scleroderma following ultraviolet-A1 irradiation. J Am Acad Dermatol. 2007;56(6):956–9.
Sawada H, Isogai Z, Morita A. Altered decorin expression of systemic sclerosis by UVA1 (340–400 nm) phototherapy: immunohistochemical analysis of 3 cases. BMC Dermatol. 2003;3:2.
Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–16.
Kiss M, Wlaschek M, Brenneisen P, Michel G, Hommel C, Lange TS, Peus D, Kemeny L, Dobozy A, Scharffetter-Kochanek K. Alpha-melanocyte stimulating hormone induces collagenase/matrix metalloproteinase-1 in human dermal fibroblasts. Biol Chem Hoppe Seyler. 1995;376(7):425–30.
Kreuter A, Breuckmann F, Uhle A, Brockmeyer N, Von Kobyletzki G, Freitag M, Stuecker M, Hoffmann K, Gambichler T, Altmeyer P. Low-dose UVA1 phototherapy in systemic sclerosis: effects on acrosclerosis. J Am Acad Dermatol. 2004;50(5):740–7.
Wang F, Garza LA, Cho S, Kafi R, Hammerberg C, Quan T, Hamilton T, Mayes M, Ratanatharathorn V, Voorhees JJ, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Arch Dermatol. 2008;144(7):851–8.
De Rie MA, Bos JD. Photochemotherapy for systemic and localized scleroderma. J Am Acad Dermatol. 2000;43(4):725–6.
Camacho NR, Sanchez JE, Martin RF, Gonzalez JR, Sanchez JL. Medium-dose UVA1 phototherapy in localized scleroderma and its effect in CD34-positive dendritic cells. J Am Acad Dermatol. 2001;45(5):697–9.
Vielhaber G, Grether-Beck S, Koch O, Johncock W, Krutmann J. Sunscreens with an absorption maximum of > or = 360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts. Photochem Photobiol Sci. 2006;5(3):275–82.
Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, Imaizumi K, Hashimoto N, Shimokata K, Hasegawa Y, Kawabe T. Attenuation of transforming growth factor-β-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol. 2011;44(5):614–20.
Breuckmann F, Stuecker M, Altmeyer P, Kreuter A. Modulation of endothelial dysfunction and apoptosis: UVA1-mediated skin improvement in systemic sclerosis. Arch Dermatol Res. 2004;296(5):235–9.
Breuckmann F, Appelhans C, Bastian A, Stuecker M, Altmeyer P, Kreuter A. UVA1-induced decrease in dermal neuron-specific enolase (NSE) in acrosclerosis. Arch Dermatol Res. 2004;296(4):182–4.
Bulat V, Situm M, Dediol I, Ljubicic I, Bradic L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol. 2011;35(Suppl 2):147–51.
Brenneisen P, Wlaschek M, Wenk J, Blaudschun R, Hinrichs R, Dissemond J, Krieg T, Scharffetter-Kochanek K. Ultraviolet-B induction of interstitial collagenase and stromelyin-1 occurs in human dermal fibroblasts via an autocrine interleukin-6-dependent loop. FEBS Lett. 1999;449(1):36–40.
Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293(11):576–83.
Kondo S, Kooshesh F, Sauder DN. Penetration of keratinocyte-derived cytokines into basement membrane. J Cell Physiol. 1997;171(2):190–5.
Usmani N, Murphy A, Veale D, Goulden V, Goodfield M. Photochemotherapy for systemic sclerosis: effect on clinical and molecular markers. Clin Exp Dermatol. 2010;35(6):608–13.
Usmani N, Murphy A, Veale D, Goulden V, Goodfield M. Photochemotherapy for localized morphoea: effect on clinical and molecular markers. Clin Exp Dermatol. 2008;33(6):698–704.
Inoue T, Yamaoka T, Murota H, Yokomi A, Tanemura A, Igawa K, Tani M, Katayama I. Effective oral psoralen plus ultraviolet A therapy for digital ulcers with revascularization in systemic sclerosis. Acta Derm Venereol. 2014;94(2):250–1.
Brinckmann J, Neess CM, Gaber Y, Sobhi H, Notbohm H, Hunzelmann N, Fietzek PP, Muller PK, Risteli J, Gebker R, et al. Different pattern of collagen cross-links in two sclerotic skin diseases: lipodermatosclerosis and circumscribed scleroderma. J Invest Dermatol. 2001;117(2):269–73.
Sakakibara N, Sugano S, Morita A. Ultrastructural changes induced in cutaneous collagen by ultraviolet-A1 and psoralen plus ultraviolet A therapy in systemic sclerosis. J Dermatol. 2008;35(2):63–9.
Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Influence of 5-aminolevulinic acid and red light on collagen metabolism of human dermal fibroblasts. J Invest Dermatol. 2003;120(2):325–31.
Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br J Dermatol. 2004;151(4):776–83.
Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130(1):259–69.
Casara C, Eidt L, Cunha V. Prevalence study of dermatoses referred to the phototherapy unit at the Dermatology Service of the Clinics Hospital of Porto Alegre, RS, Brazil. An Bras Dermatol. 2013;88(2):211–5.
Warburton KL, McPhee MJ, Savage LJ, Honan AE, Montgomery R, Ghazavi M, Torley D, Shams K, Ingram JR. Management of morphoea: results of a national survey of UK clinicians. Br J Dermatol. 2014;171(5):1243–5.
Johnson W, Jacobe H. Morphea in adults and children cohort II: patients with morphea experience delay in diagnosis and large variation in treatment. J Am Acad Dermatol. 2012;67(5):881–9.
Hawley DP, Pain CE, Baildam EM, Murphy R, Taylor AE, Foster HE. United Kingdom survey of current management of juvenile localized scleroderma. Rheumatology (Oxford). 2014;53(10):1849–54.
Strickland N, Patel G, Strickland A, Jacobe H. Attitudes and trends in the treatment of morphea: a national survey. J Am Acad Dermatol. 2015;72(4):727–8.
Kerscher M, Volkenandt M, Meurer M, Lehmann P, Plewig G, Röcken M. Treatment of localised scleroderma with PUVA bath photochemotherapy. Lancet. 1994;343(8907):1233.
Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy. Lancet. 1995;346(8983):1166.
Kroft EB, van de Kerkhof PC, Gerritsen MJ, de Jong EM. Period of remission after treatment with UVA-1 in sclerodermic skin diseases. J Eur Acad Dermatol Venereol. 2008;22(7):839–44.
Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium-dose is more effective than low-dose ultraviolet Al phototherapy for localized scleroderma as shown by 20-MHz ultrasound assessment. J Am Acad Dermatol. 2009;60(5):786–91.
Su O, Onsun N, Onay HK, Erdemoglu Y, Ozkaya DB, Cebeci F, Somay A. Effectiveness of medium-dose ultraviolet Al phototherapy in localized scleroderma. Int J Dermatol. 2011;50(8):1006–13.
Kerscher M, Volkenandt M, Gruss C, Reuther T, von Kobyletzki G, Freitag M, Dirschka T, Altmeyer P. Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol. 1998;38(1):21–6.
Jacobe HT, Cayce R, Nguyen J. UVA1 phototherapy is effective in darker skin: a review of 101 patients of Fitzpatrick skin types I–V. Br J Dermatol. 2008;159(3):691–6.
Connolly KL, Griffith JL, McEvoy M, Lim HW. Ultraviolet A1 phototherapy beyond morphea: experience in 83 patients. Photodermatol Photoimmunol Photomed. 2015;31(6):289–95.
Suh KS, Kang JS, Baek JW, Kim TK, Lee JW, Jeon YS, Jang MS, Kim ST. Efficacy of ultraviolet A1 phototherapy in recalcitrant skin diseases. Ann Dermatol. 2010;22(1):1–8.
El-Mofty M, Mostafa W, El-Darouty M, Bosseila M, Nada H, Yousef R, Esmat S, El-Lawindy M, Assaf M, El-Enani G. Different low doses of broad-band UVA in the treatment of morphea and systemic sclerosis. Photodermatol Photoimmunol Photomed. 2004;20(3):148–56.
Vasquez R, Jabbar A, Khan F, Buethe D, Ahn C, Jacobe H. Recurrence of morphea after successful ultraviolet A1 phototherapy: a cohort study. J Am Acad Dermatol. 2014;70(3):481–8.
Ridge CA, Moktar A, Barry J, Murphy GM. Photochemotherapy and methotrexate used to treat generalized cutaneous scleroderma. J Eur Acad Dermatol Venereol. 2007;21(5):692–3.
Kerscher M, Meurer M, Sander C, Volkenandt M, Lehmann P, Plewig G, Rocken M. PUVA bath photochemotherapy for localized scleroderma. Evaluation of 17 consecutive patients. Arch Dermatol. 1996;132(11):1280–2.
Newland K, Marshman G. Success treatment of post-irradiation morphoea with acitretin and narrowband UVB. Australas J Dermatol. 2012;53(2):136–8.
Buense R, Duarte IA, Bouer M. Localized scleroderma: assessment of the therapeutic response to phototherapy. An Bras Dermatol. 2012;87(1):63–9.
Barolet D. Pulsed versus continuous wave low-level light therapy on osteoarticular signs and symptoms in limited scleroderma (CREST syndrome): a case report. J Biomed Opt. 2014;19(11):118001.
Nistico SP, Saraceno R, Schipani C, Costanzo A, Chimenti S. Different applications of monochromatic excimer light in skin diseases. Photomed Laser Surg. 2009;27(4):647–54.
Kineston D, Kwan JM, Uebelhoer NS, Shumaker PR. Use of a fractional ablative 10.6-μm carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147(10):1148–50.
Comstedt LR, Svensson A, Troilius A. Improvement of microstomia in scleroderma after intense pulsed light: a case series of four patients. J Cosmet Laser Ther. 2012;14(2):102–6.
Batchelor R, Lamb S, Goulden V, Stables G, Goodfield M, Merchant W. Photodynamic therapy for the treatment of morphoea. Clin Exp Dermatol. 2008;33(5):661–3.
Breuckmann F, Gambichler T, Altmeyer P, Kreuter A. UVA/UVA1 phototherapy and PUVA photochemotherapy in connective tissue diseases and related disorders: a research based review. BMC Dermatol. 2004;4(1):11.
Sacher C, Konig C, Scharffetter-Kochanek K, Krieg T, Hunzelmann N. Bullous pemphigoid in a patient treated with UVA-1 phototherapy for disseminated morphea. Dermatology. 2001;202(1):54–7.
Kreuter A, Gambichler T, Avermaete A, Jansen T, Hoffmann M, Hoffmann K, Altmeyer P, von Kobyletzki G, Bacharach-Buhles M. Combined treatment with calcipotriol ointment and low-dose ultraviolet A1 phototherapy in childhood morphea. Pediatr Dermatol. 2001;18(3):241–5.
Lam J, Polifka JE, Dohil MA. Safety of dermatologic drugs used in pregnant patients with psoriasis and other inflammatory skin diseases. J Am Acad Dermatol. 2008;59(2):295–315.
Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148(1):132–3.
Borradale D, Isenring E, Hacker E, Kimlin MG. Exposure to solar ultraviolet radiation is associated with a decreased folate status in women of childbearing age. J Photochem Photobiol B. 2014;131:90–5.
Bielsa Marsol I. Update on the classification and treatment of localized scleroderma. Actas Dermosifiliogr. 2013;104(8):654–66.
Durand F, Staumont D, Bonnevalle A, Hachulla E, Hatron PY, Thomas P. Ultraviolet Al phototherapy for treatment of acrosclerosis in systemic sclerosis: controlled study with half-side comparison analysis. Photodermatol Photoimmunol Photomed. 2007;23(6):215–21.
Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54(3):440–7.
de Rie MA, Enomoto DN, de Vries HJ, Bos JD. Evaluation of medium-dose UVA1 phototherapy in localized scleroderma with the cutometer and fast Fourier transform method. Dermatology. 2003;207(3):298–301.
Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, Boedeker R, Diepgen T, Dierks K, Goerz G, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. 1997;36(6 Pt 1):938–44.
Andres C, Kollmar A, Mempel M, Hein R, Ring J, Eberlein B. Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol. 2010;162(2):445–7.
Gruss CJ, Von Kobyletzki G, Behrens-Williams SC, Lininger J, Reuther T, Kerscher M, Altmeyer P. Effects of low dose ultraviolet A-1 phototherapy on morphea. Photodermatol Photoimmunol Photomed. 2001;17(4):149–55.
Morita A, Kobayashi K, Isomura I, Tsuji T, Krutmann J. Ultraviolet A1 (340–400 nm) phototherapy for scleroderma in systemic sclerosis. J Am Acad Dermatol. 2000;43(4):670–4.
Grundmann-Kollmann M, Ochsendorf F, Zollner TM, Spieth K, Sachsenberg-Studer E, Kaufmann R, Podda M. PUVA-cream photochemotherapy for the treatment of localized scleroderma. J Am Acad Dermatol. 2000;43(4):675–8.
Hofer A, Soyer HP. Oral psoralen-UV-A for systemic scleroderma. Arch Dermatol. 1999;135(5):603–4.
Karrer S, Abels C, Landthaler M, Szeimies RM. Topical photodynamic therapy for localized scleroderma. Acta Derm Venereol. 2000;80(1):26–7.
Rose RF, Turner D, Goodfield MJ, Goulden V. Low-dose UVA1 phototherapy for proximal and acral scleroderma in systemic sclerosis. Photodermatol Photoimmunol Photomed. 2009;25(3):153–5.
Pereira N, Santiago F, Oliveira H, Figueiredo A. Low-dose UVA(1) phototherapy for scleroderma: what benefit can we expect? J Eur Acad Dermatol Venereol. 2012;26(5):619–26.
von Kobyletzki G, Uhle A, Pieck C, Hoffmann K, Altmeyer P. Acrosclerosis in patients with systemic sclerosis responds to low-dose UV-A1 phototherapy. Arch Dermatol. 2000;136(2):275–6.
Oikarinen A, Knuutinen A. Ultraviolet A sunbed used for the treatment of scleroderma. Acta Derm Venereol. 2001;81(6):432–3.
Rombold S, Lobisch K, Katzer K, Grazziotin TC, Ring J, Eberlein B. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24(1):19–23.
Kreuter A, Hyun J, Skrygan M, Sommer A, Tomi NS, Breuckmann F, Altmeyer P, Gambichler T. Ultraviolet A1 phototherapy decreases inhibitory SMAD7 gene expression in localized scleroderma. Arch Dermatol Res. 2006;298(6):265–72.
Tuchinda C, Kerr HA, Taylor CR, Jacobe H, Bergamo BM, Elmets C, Rivard J, Lim HW. UVA1 phototherapy for cutaneous diseases: an experience of 92 cases in the United States. Photodermatol Photoimmunol Photomed. 2006;22(5):247–53.
Ozdemir M, Engin B, Toy H, Mevlitoglu I. Treatment of plaque-type localized scleroderma with retinoic acid and ultraviolet A plus the photosensitizer psoralen: a case series. J Eur Acad Dermatol Venereol. 2008;22(4):519–21.
Pavlotsky F, Sakka N, Lozinski A, Barzilai A. Bath psoralen-UVA photochemotherapy for localized scleroderma: experience from a single institute. Photodermatol Photoimmunol Photomed. 2013;29(5):247–52.
Morison WL. Psoralen UVA therapy for linear and generalized morphea. J Am Acad Dermatol. 1997;37(4):657–9.
Pasic A, Ceovic R, Lipozencic J, Husar K, Susic SM, Skerlev M, Hrsan D. Phototherapy in pediatric patients. Pediatr Dermatol. 2003;20(1):71–7.
von Felbert V, Kernland-Lang K, Hoffmann G, Wienert V, Simon D, Hunziker T. Irradiation with water-filtered infrared A plus visible light improves cutaneous scleroderma lesions in a series of cases. Dermatology. 2011;222(4):347–57.
Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218(1):63–6.
Uchiyama M, Okubo Y, Kawashima H, Yamamoto K, Mitsuhashi Y, Tsuboi R. Case of localized scleroderma successfully treated with bath psoralen and ultraviolet A therapy. J Dermatol. 2010;37(1):75–80.
Morita A, Sakakibara S, Sakakibara N, Yamauchi R, Tsuji T. Successful treatment of systemic sclerosis with topical PUVA. J Rheumatol. 1995;22(12):2361–5.
Mohanna M, Distler O, Sprott H, Kündig T, French LE, Hofbauer G. Skin lesions in anti-Pm-Scl-70 positive systemic sclerosis-dermatomyositis overlap syndrome improve during local PUVA phototherapy. Eur J Dermatol. 2013;23(5):730–1.
Kanekura T, Fukumaru S, Matsushita S, Terasaki K, Mizoguchi S, Kanzaki T. Successful treatment of scleroderma with PUVA therapy. J Dermatol. 1996;23(7):455–9.
García-Bustínduy M, Noda A, Sánchez R, González de Mesa MJ, Guimerá F, García-Montelongo R. PUVA therapy in localized scleroderma. J Eur Acad Dermatol Venereol. 1998;10(3):283–4.
Todd DJ, Askari A, Ektaish E. PUVA therapy for disabling pansclerotic morphoea of children. Br J Dermatol. 1998;138(1):201–2.
Baum S, Pavlotsky F, Shpiro D, Trau H. PUVA treatment in sclerodermatoid spectrum of dermatologic diseases: our initial experience. Isr Med Assoc J. 2004;6(9):563–4.
Rose RF, Goodfield MJ. Combining PUVA therapy with systemic immunosuppression to treat progressive diffuse morphoea. Clin Exp Dermatol. 2005;30(3):226–8.
Gambichler T, Kreuter A, Rotterdam S, Altmeyer P, Hoffmann K. Linear scleroderma 'en coup de sabre' treated with topical calcipotriol and cream psoralen plus ultraviolet A. J Eur Acad Dermatol Venereol. 2003;17(5):601–2.
Aragane Y, Kawada A, Maeda A, Isogai R, Isogai N, Tezuka T. Disseminated scleroderma of a Japanese patient successfully treated with bath PUVA photochemotherapy. J Cutan Med Surg. 2001;5(2):135–9.
Yamaguchi K, Takeuchi I, Yoshii N, Gushi A, Kanekura T, Kanzaki T. The discrepancy in hardness between clinical and histopathological findings in localized scleroderma treated with PUVA. J Dermatol. 1998;25(8):544–6.
Wollina U, Looks A, Uhlemann C, Wollina K. Pansclerotic morphea of childhood-follow-up over 6 years. Pediatr Dermatol. 1999;16(3):245–7.
Lim D, Johnston S, Novakovic L, Fearfield L. Radiation-induced morphoea treated with UVA-1 phototherapy. Clin Exp Dermatol. 2014;39(5):612–5.
Scharffetter-Kochanek K, Goldermann R, Lehmann P, Holzle E, Goerz G. PUVA therapy in disabling pansclerotic morphoea of children. Br J Dermatol. 1995;132(5):830–1.
Yildirim M, Baysal V, Aridogan BC, Kesici D, Erturan I. Pansclerotic morphea treated with UVA: a case report. J Dermatol. 2003;30(8):625–7.
Tewari A, Garibaldinos T, Lai-Cheong J, Groves R, Sarkany R, Branislav Novakovic L. Successful treatment of microstomia with UVA1 phototherapy in systemic sclerosis. Photodermatol Photoimmunol Photomed. 2011;27(2):113–4.
Forsea AM, Cretu AN, Ionescu R, Giurcaneanu C. Disabling pansclerotic morphea of childhood–unusual case and management challenges. J Med Life. 2008;1(3):348–54.
Herzinger T, Prinz JC, Röcken M. Pansclerotic morphea of the head. Arch Dermatol. 2008;144(1):125–6.
Gruss C, Stucker M, Kobyletzki G, Schreiber D, Altmeyer P, Kerscher M. Low dose UVA1 phototherapy in disabling pansclerotic morphoea of childhood. Br J Dermatol. 1997;136(2):293–4.
Steger JW, Matthews JH. UVA therapy for scleroderma. J Am Acad Dermatol. 1999;40(5 Pt 1):787–8.
Brownell I, Soter NA, Franks AG Jr. Familial linear scleroderma (en coup de sabre) responsive to antimalarials and narrowband ultraviolet B therapy. Dermatol Online J. 2007;13(1):11.
Cho S, Choi MJ, Zheng Z, Goo B, Kim DY, Cho SB. Clinical effects of non-ablative and ablative fractional lasers on various hair disorders: a case series of 17 patients. J Cosmet Laser Ther. 2013;15(2):74–9.
Eisen D, Alster TS. Use of a 585 nm pulsed dye laser for the treatment of morphea. Dermatol Surg. 2002;28(7):615–6.
Acknowledgments
No funding or sponsorship was received for publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
John Hassani and Steven R. Feldman have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C7E4F0600C13C58F.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hassani, J., Feldman, S.R. Phototherapy in Scleroderma. Dermatol Ther (Heidelb) 6, 519–553 (2016). https://doi.org/10.1007/s13555-016-0136-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-016-0136-3