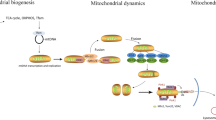
Abstract
The pathophysiological mechanisms underpinning the development of, and recovery from, sepsis-induced organ failure require further delineation. Mitochondrial dysfunction may well play a key role. This review will therefore consider mitochondria’s function in normal physiology, evidence linking bioenergetic alterations to organ dysfunction after severe and prolonged inflammation, and potential therapeutic strategies that may be applied.
Résumé
Au cours du sepsis, les mécanismes physiopathologiques sous-tendant le développement et la récupération des dysfonctions d’organe sont encore mal compris. La dysfonction mitochondriale pourrait jouer un rôle majeur. Cette mise au point décrit la physiologie normale des mitochondries, les arguments reliant l’altération bioénergétique mitochondriale aux dysfonctions d’organes après des périodes d’inflammation sévère et prolongée. Enfin, les perspectives thérapeutiques envisageables seront abordées.
Similar content being viewed by others
References
Alberti C, Brun-Buisson C, Burchardi H, et al (2002) Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med 28:108–121
Angus DC, Linde-Zwirble WT, Lidicker J, et al (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Brealey D, Brand M, Hargreaves I, et al (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223
Duchen MR (2004) Roles of mitochondria in health and disease. Diabetes 53(Suppl 1):S96–S102
Rich P (2003) Chemiosmotic coupling: The cost of living. Nature 421:583
Bradley SJ, Kingwell BA, McConell GK (1999) Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48:1815–1821
Evans MJ, Scarpulla RC (1990) NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev 4:1023–1034
Puigserver P, Wu Z, Park CW, et al (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Scarpulla RC (2002) Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286:81–89
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A 91:1309–1313
Akimoto T, Pohnert SC, Li P, et al (2005) Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280:19587–19593
Czubryt MP, McAnally J, Fishman GI, et al (2003) Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A 100:1711–1716
Schaeffer PJ, Wende AR, Magee CJ, et al (2004) Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem 279:39593–39603
Zong H, Ren JM, Young LH, et al (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 99:15983–15987
Gerhart-Hines Z, Rodgers JT, Bare O, et al (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J 26:1913–1923
Fan M, Rhee J, St-Pierre J, et al (2004) Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev 18:278–289
Leonardsson G, Steel JH, Christian M, et al (2004) Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A 101:8437–8442
Lerin C, Rodgers JT, Kalume DE, et al (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab 3:429–438
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79:208–217
Nisoli E, Clementi E, Paolucci C, et al (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899
Nisoli E, Carruba MO (2006) Nitric oxide and mitochondrial biogenesis. J Cell Sci 119:2855–2862
Liesa M, Palacin M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845
Glancy B, Balaban RS (2011) Protein composition and function of red and white skeletal muscle mitochondria. Am J Physiol Cell Physiol 300:C1280–C1290
Benard G, Faustin B, Passerieux E, et al (2006) Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol 291:C1172–C1182
Collins TJ, Berridge MJ, Lipp P, et al (2002) Mitochondria are morphologically and functionally heterogeneous within cells. Embo J 21:1616–1627
Rivers E, Nguyen B, Havstad S, et al (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Gattinoni L, Brazzi L, Pelosi P, et al (1995) A trial of goaloriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 333:1025–1032
Hayes MA, Timmins AC, Yau EH, et al (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330:1717–1722
Fink MP (2002) Bench-to-bedside review: Cytopathic hypoxia. Crit Care 6:491–499
Kreymann G, Grosser S, Buggisch P, et al (1993) Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med 21:1012–1019
Boekstegers P, Weidenhofer S, Pilz G, et al (1991) Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection 19:317–323
Rosser DM, Stidwill RP, Jacobson D, et al (1995) Oxygen tension in the bladder epithelium rises in both high and low cardiac output endotoxemic sepsis. J Appl Physiol 79:1878–1882
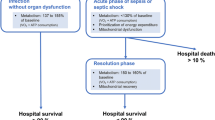
Singer M (2007) Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 35:S441–S448
Brealey D, Karyampudi S, Jacques TS, et al (2004) Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286:R491–R497
Comim CM, Rezin GT, Scaini G, et al (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318
d’Avila JC, Santiago AP, Amancio RT, et al (2008) Sepsis induces brain mitochondrial dysfunction. Crit Care Med 36:1925–1932
Gellerich FN, Trumbeckaite S, Hertel K, et al (1999) Impaired energy metabolism in hearts of septic baboons: diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock 11:336–3341
Adrie C, Bachelet M, Vayssier-Taussat M, et al (2001) Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med 164:389–395
Callahan LA, Supinski GS (2005) Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 172:861–868
Carre JE, Orban JC, Re L, et al (2010) Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 182:745–751
Fredriksson K, Hammarqvist F, Strigard K, et al (2006) Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab 291:E1044–F1050
Vanhorebeek I, De Vos R, Mesotten D, et al (2005) Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 365:53–59
Fredriksson K, Flaring U, Guillet C, et al (2009) Muscle mitochondrial activity increases rapidly after an endotoxin challenge in human volunteers. Acta Anaesthesiol Scand 53:299–304
Clementi E, Brown GC, Feelisch M, et al (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of Snitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A 95:7631–7636
Murray J, Taylor SW, Zhang B, et al (2003) Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem 278:37223–37230
Galley HF (2011) Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 107:57–64
Hotchkiss RS, Song SK, Neil JJ, et al (1991) Sepsis does not impair tricarboxylic acid cycle in the heart. Am J Physiol 260: C50–C57
Vary TC (1996) Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock 6:89–94
Mason KE, Stofan DA (2008) Endotoxin challenge reduces aconitase activity in myocardial tissue. Arch Biochem Biophys 469:151–156
Calvano SE, Xiao W, Richards DR, et al (2005) A network-based analysis of systemic inflammation in humans. Nature 437:1032–1037
Fredriksson K, Tjader I, Keller P, et al (2008) Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One 3:e3686
Haden DW, Suliman HB, Carraway MS, et al (2007) Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med 176:768–777
Mofarrahi M, Sigala I, Guo Y, et al (2012) Autophagy and skeletal muscles in sepsis. PLoS One 7:e47265
Ritter C, Andrades ME, Reinke A, et al (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Lowes DA, Thottakam BM, Webster NR, et al (2008) The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45:1559–1565
Mao G, Kraus GA, Kim I, et al (2010) A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J Nutr 140:1425–1431
Supinski GS, Murphy MP, Callahan LA (2009) MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 297:R1095–R1102
Escames G, Leon J, Macias M, et al (2003) Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. Faseb J 17:932–934
Mundigler G, Delle-Karth G, Koreny M, et al (2002) Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 30:536–540
Gitto E, Pellegrino S, Gitto P, et al (2009) Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J Pineal Res 46:128–139
Galley HF (2010) Bench-to-bedside review: Targeting antioxidants to mitochondria in sepsis. Crit Care 14:230
Ryter SW, Choi AM (2007) Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp 280:165–175; discussion 175–81
Lancel S, Hassoun SM, Favory R, et al (2009) Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther 329:641–648
MacGarvey NC, Suliman HB, Bartz RR, et al (2012) Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NFE2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med 185:851–861
Piantadosi CA, Withers CM, Bartz RR, et al (2011) Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286: 16374–16385
Blackstone E, Morrison M, Roth MB (2005) H2S induces a suspended animation-like state in mice. Science 308:518
Spiller F, Orrico MI, Nascimento DC, et al (2010) Hydrogen sulfide improves neutrophil migration and survival in sepsis via K +ATP channel activation. Am J Respir Crit Care Med 182:360–368
Tokuda K, Kida K, Marutani E, et al (2012) Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal 17:11–21
Thomas RR, Khan SM, Portell FR, et al (2011) Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion 11:108–118
Herridge MS, Tansey CM, Matte A, et al (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364:1293–1304
Khan J, Harrison TB, Rich MM, et al (2006) Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 67:1421–1425
Kress JP, Herridge MS (2012) Medical and economic implications of physical disability of survivorship. Semin Respir Crit Care Med 33:339–347
Author information
Authors and Affiliations
Corresponding author
Additional information
Cet article correspond à la conférence faite par l’auteur au congrès de la SRLF 2013 dans la session: Voies de recherche dans le sepsis.
Rights and permissions
About this article
Cite this article
Saeed, S., Singer, M. Mitochondria — key roles in sepsis. Réanimation 22 (Suppl 2), 352–358 (2013). https://doi.org/10.1007/s13546-012-0638-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13546-012-0638-7