Abstract
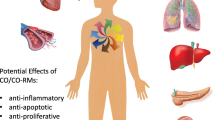
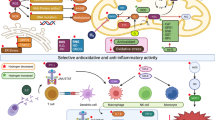
Small amounts of carbon monoxide (CO) are continuously produced in mammals. The intracellular levels of this gaseous molecule can markedly increase under stressful conditions following the induction of heme oxygenases (HO), ubiquitous enzymes responsible for the catabolism of heme. The development of a technology concerning the CO-releasing molecules (CO-RMs) that control the delivery and action of CO under different pathological conditions represents a major step forward in the development of CO-based pharmaceuticals with therapeutic applications. CO is important for the homeostatic control of cardiovascular functions. Abnormal metabolism and function of CO contribute to the pathogenesis and development of hypertension. Another vascular disease in which the role of CO has been evaluated is pulmonary arterial hypertension. Important results have been reported in which CO prevents intimal hyperplasia by arresting hyperproliferative vascular smooth muscle cells as well as increased mobilization and recruitment of bone-marrow-derived progenitor cells. Transplantation has been a field of research, in which most studies have investigated the beneficial properties of CO-RMs. CO gas and CO-RMs have produced promising results in the preservation of organs for transplantation. The anti-inflammatory properties of CO and CO-RMs have been demonstrated in a multitude of animal models of inflammation, suggesting a possible therapeutic application for inflammatory diseases. Despite therapeutic benefit in animal model studies, the efficacy of CO in humans remains unclear. Further studies are expected to better understand the pharmacokinetics as well as long- and short-term effects of CO-RMs.
Résumé
Du monoxyde de carbone (CO) est continuellement produit en petites quantités chez les mammifères. Le niveau intracellulaire de cette molécule gazeuse augmente de façon significative en conditions de stress, après induction des hèmes oxygénases (HO), enzymes ubiquitaires responsables du catabolisme de l’hème. Pouvoir disposer de molécules libérant du CO (MLCO), avec la possibilité de maîtrise de ses conditions de production et d’action, représente une étape essentielle pour le développement de médicaments à base de CO pouvant déboucher sur des applications thérapeutiques. Le CO joue un rôle important dans le contrôle homéostatique des fonctions cardiovasculaires. Toute altération du métabolisme ou de l’utilisation du CO peut contribuer au développement d’une hypertension. L’hypertension artérielle pulmonaire est l’une de ces conditions pathologiques où le CO joue un rôle mécanistique significatif. Des données importantes ont été publiées, démontrant que le CO prévient l’hyperplasie intimale en stoppant la prolifération des cellules musculaires lisses et en inhibant l’augmentation de mobilisation et de recrutement de cellules souches progénitrices dérivant de la moelle osseuse. La transplantation est un domaine de recherche où plusieurs études ont montré le bénéfice de ces MLCO. Le CO et les MLCO sont à l’origine de résultats prometteurs dans le champ de la préservation des organes pour la greffe. Les propriétés anti-inflammatoires du CO et des MLCO ont été retrouvées dans de nombreuses études, au travers de modèles animaux d’inflammation, suggérant ainsi de possibles applications dans le traitement des pathologies inflammatoires. Néanmoins et malgré un bénéfice thérapeutique établi expérimentalement, l’intérêt du CO chez l’homme reste incertain. Des études à venir sont encore attendues pour permettre un progrès des connaissances tant pour la pharmacocinétique que pour les effets à court et à long terme des MLCO.
Similar content being viewed by others
References
Kajimura M, Fukuda R, Bateman RM, et al (2010) Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal 13:157–192
Piantadosi CA, Tatro L, Zhang J (1995) Hydroxyl radical production in the brain after CO hypoxia in rats. Free Radic Biol Med 18:603–609
Song R, Zhou Z, Kim PK, et al (2004) Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J Biol Chem 279:44327–44334
Wu L, Wang R (2005) Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57:585–630
Ryter SW, Otterbein LE (2004) Carbon monoxide in biology and medicine. Bioessays 26:270–280
Cooper CE, Brown GC (2008) The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40:533–539
Wilkinson WJ, Kemp PJ (2011) Carbon monoxide: an emerging regulator of ion channels. J Physiol 589:3055–3062
Wilks A, Medzihradszky KF, Ortiz de Montellano PR (1998) Heme oxygenase active-site residues identified by heme-protein cross-linking during reduction of CBrCl3. Biochemistry 37:2889–2896
Morse D, Lin L, Choi AM, et al (2009) Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med 47:1–12
Young LJ, Caughey WS (1986) Oxygenation of carbon monoxide by bovine heart cytochrome c oxidase. Biochemistry 25:152–161
Motterlini R, Mann BE, Johnson TR, et al (2003) Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des 9:2525–2539
Vergely C, Maupoil V, Clermont G, et al (2003) Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch Biochem Biophys 420:209–216
Vergely C, Perrin-Sarrado C, Clermont G, et al (2002) Postischemic recovery and oxidative stress are independent of nitric oxide synthases modulation in isolated rat heart. J Pharmacol Exp Therap 303:149–157
Thom SR, Fisher D, Xu YA, et al (2000) Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc Natl Acad Sci U S A 97:1305–1310
Wang X, Wang Y, Kim HP, et al (2007) Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282:1718–1726
Rhodes MA, Carraway MS, Piantadosi CA, et al (2009) Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am J Physiol Heart Circ Physiol 297: H392–H399
Lee SJ, Ryter SW, Xu J, et al (2011) Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol 45:867–873
Watts RN, Ponka P, Richardson DR (2003) Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron. Biochem J 369:429–440
Piantadosi CA (2002) Biological chemistry of carbon monoxide. Antioxid Redox Signal 4:259–270
Motterlini R, Otterbein LE (2010) The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 9:728–743
Fan W, Huang F, Wu Z, et al (2011) Carbon monoxide: a gas that modulates nociception. J Neurosci Res 89:802–807
Motterlini R (2007) Carbon monoxide-releasing molecules (CORMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans 35:1142–1146
Chan KH, Ng MK, Stocker R (2011) Haem oxygenase-1 and cardiovascular disease: mechanisms and therapeutic potential. Clin Sci (Lond) 120:493–504
Ndisang JF, Tabien HE, Wang R (2004) Carbon monoxide and hypertension. J Hypertens 22:1057–1074
Mustafa MR, Johns EJ (2001) The role of haem oxygenase in renal vascular reactivity in normotensive and hypertensive rats. J Hypertens 19:1105–1111
Aizawa T, Ishizaka N, Taguchi J, et al (2000) Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension 35:800–806
Chen B, Guo L, Fan C, et al (2009) Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol 175:422–429
Chlopicki S, Olszanecki R, Marcinkiewicz E, et al (2006) Carbon monoxide released by CORM-3 inhibits human platelets by a mechanism independent of soluble guanylate cyclase. Cardiovasc Res 71:393–401
Clark JE, Naughton P, Shurey S, et al (2003) Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res 93:e2–e8
Varadi J, Lekli I, Juhasz B, et al (2007) Beneficial effects of carbon monoxide-releasing molecules on post-ischemic myocardial recovery. Life Sci 80:1619–1626
Lo Iacono L, Boczkowski J, Zini R, et al (2011) A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Radic Biol Med 50:1556–1564
Wegiel B, Gallo DJ, Raman KG, et al (2010) Nitric oxidedependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation 121:537–548
Sato K, Balla J, Otterbein L, et al (2001) Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 166:4185–4194
Bagul A, Hosgood SA, Kaushik M, et al (2008) Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheart beating donor kidney. Transplantation 85:576–581
Amersi F, Shen XD, Anselmo D, et al (2002) Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology 35:815–823
Fujita T, Toda K, Karimova A, et al (2001) Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med 7:598–604
Brouard S, Otterbein LE, Anrather J, et al (2000) Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192:1015–1026
Zhou Z, Song R, Fattman CL, et al (2005) Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol 166:27–37
Zhang X, Shan P, Alam J, et al (2003) Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 278:22061–22070
Zhang X, Shan P, Alam J, et al (2005) Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem 280:8714–8721
Chung SW, Liu X, Macias AA, et al (2008) Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118:239–247
Ryter SW, Choi AM (2010) Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr Drug Targets 11:1485–1494
Author information
Authors and Affiliations
Corresponding author
Additional information
Cet article correspond à la conférence faite par l’auteur au congrès de la SRLF 2012 dans la session: Plein gaz.
Rights and permissions
About this article
Cite this article
Rochette, L., Vergely, C., Rochette, F. et al. Carbon monoxide: a new pharmaceutical agent?. Réanimation 21 (Suppl 2), 460–466 (2012). https://doi.org/10.1007/s13546-011-0430-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13546-011-0430-4