Abstract
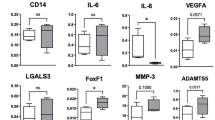
This study aimed to evaluate the inhibitory effects of micro-current stimulation (MCS) on inflammatory responses in chondrocytes and degradation of extracellular matrix (ECM) in osteoarthritis (OA). To determine the efficacy of MCS, IL-1β-treated chondrocytes and monosodium iodoacetate (MIA)-induced OA rat model were used. To evaluate the cytotoxicity and nitric oxide (NO) production in SW1353 cells, the presence or absence of IL-1β treatment or various levels of MCS were applied. Immunoblot analysis was conducted to evaluate whether MCS can modulate IL-1R1/MyD88/NF-κB signaling pathway and various indicators involved in ECM degradation. Additionally, to determine whether MCS alleviates subchondral bone structure destruction caused by OA, micro-CT analysis, immunoblot analysis, and ELISA were conducted using OA rat model. 25 and 50 µA levels of MCS showed effects in cell proliferation and NO production. The MCS group with IL-1β treatment lead to significant inhibition of protein expression levels regarding IL-1R1/MyD88/NF-κB signaling and reduction of the nucleus translocation of NF-κB. In addition, the protein expression levels of MMP-1, MMP-3, MMP-13, and IL-1β decreased, whereas collagen II and aggrecan increased. In animal results, morphological analysis of subchondral bone using micro-CT showed that MCS induced subchondral bone regeneration and improvement, as evidenced by increased thickness and bone mineral density of the subchondral bone. Furthermore, MCS-applied groups showed decreases in the protein expression of MMP-1 and MMP-3, while increases in collagen-II and aggrecan expressions. These findings suggest that MCS has the potential to be used as a non-pharmaceutical method to alleviate OA.






Similar content being viewed by others
References
Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthr Cartil. 2005;13:643–54.
Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilagein osteoarthritis: a review. In Proceedings of the Seminars in Arthritis and Rheumatism, 1998; pp. 392–399.
Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–25.
Kim KM, Kim YJ, Kim JM, Sohn DH, Park YC. Change in the levels of intracellular antioxidants during aging of articular chondrocytes and cartilage. J Life Sci. 2019;29:888–94.
Chen J-J, Huang J-F, Du W-X, Tong P-J. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med. 2014;7:297–300.
Chevalier X, Conrozier T, Richette P. Desperately looking for the right target in osteoarthritis: the anti-IL-1 strategy. 2011;13(4):1–2.
Sandell LJ, Xing X, Franz C, Davies S, Chang L-W, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1β. Osteoarthr Cartil. 2008;16:1560–71.
Martino MM, Maruyama K, Kuhn GA, Satoh T, Takeuchi O, Müller R, Akira S. Inhibition of IL-1R1/MyD88 signalling promotes mesenchymal stem cell-driven tissue regeneration. Nat Commun. 2016;7:11051.
Roman-Blas J, Jimenez S. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr Cartil. 2006;14:839–48.
Marcu B, Otero K, Olivotto M, Maria Borzi E, Goldring R;B. NF-κB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613.
Van Manen MD, Nace J, Mont MA. Management of primary knee osteoarthritis and indications for total knee arthroplasty for general practitioners. J Osteopath Med. 2012;112:709–15.
Lee H, Han S. The effects of swimming and aquarobics exercise programs for female elderly suffering from degenerative arthritis on leg muscular function and knee joint ROM. Korea J Sports Sci. 2010;19:1129–39.
Chung J, Cho N. Micro-current treatment effects on pain, balance of the degenerative knee arthritis. J Korean Soc Integr Med. 2015;3:9–16.
Lee H, Hwang D, Lee M, Lee J, Cho S, Kim T-J, Kim HS. Micro-current stimulation suppresses inflammatory responses in peptidoglycan-treated raw 264.7 macrophages and Propionibacterium acnes-induced skin inflammation via TLR2/NF-κB signaling pathway. Int J Mol Sci. 2022;23:2508.
Yang G, Wang K, Song H, Zhu R, Ding S, Yang H, Sun J, Wen X, Sun L. Celastrol ameliorates osteoarthritis via regulating TLR2/NF-κB signaling pathway. Front Pharmacol. 2022;13:963506.
Park S, Hwang Y, Lee H. The effect of microamerage stimulation in mice achilles tendon injury. J Korea Sprot Res. 2007;18:157–65.
Poltawski L, Watson T. Bioelectricity and microcurrent therapy for tissue healing–a narrative review. Phys Therapy Reviews. 2009;14:104–14.
Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Research®. 2004;419:30–7.
Wang Y, Simpson JA, Wluka AE, Teichtahl AJ, English DR, Giles GG, Graves S, Cicuttini FM. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res Therapy. 2009;11:1–10.
Duarte LR. The stimulation of bone growth by ultrasound. Archives Orthop Trauma Surg. 1983;101:153–9.
Bang H-s, Kang J-h, Cheon S-h, Kim M-h, Park S-j, Kim J-s, Park R-J. The effect of microcurrent electrical stimulation on the articular cartilage recovery in osteoarthritis. J Korean Soc Phys Med. 2007;2:151–9.
Love MR, Palee S, Chattipakorn SC, Chattipakorn N. Effects of electrical stimulation on cell proliferation and apoptosis. J Cell Physiol. 2018;233:1860–76.
Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–54.
Eizirik DL, Mandrup-Poulsen T. A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–33.
Henneke P, Takeuchi O, van Strijp JA, Guttormsen H-K, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL. Novel engagement of CD14 and multiple toll-like receptors by group B Streptococci. J Immunol. 2001;167:7069–76.
Choi M-C, Jo J, Park J, Kang HK, Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8:734.
Akhtar N, Khan NM, Ashruf OS, Haqqi TM. Inhibition of cartilage degradation and suppression of PGE2 and MMPs expression by pomegranate fruit extract in a model of posttraumatic osteoarthritis. Nutrition. 2017;33:1–13.
Lee H, Lee J-H, Kim D, Hwang D, Lee M, Chung H, Kim T-J, Kim HS. Micro-current stimulation can modulate the adipogenesis process by regulating the Insulin Signaling Pathway in 3T3-L1 cells and ob/ob mice. Life. 2023;13:404.
Hwang D, Lee H, Lee M, Cho S, Kim HS. The Micro-current Stimulation inhibits adipogenesis by activating Wnt/β-Catenin signaling. J Biomedical Eng Res. 2020;41:235–46.
Zhu X, Chan YT, Yung PS, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biology. 2021;8:607764.
Zhao X, Ma L, Guo H, Wang J, Zhang S, Yang X, Yang L, Jin Q. Osteoclasts secrete leukemia inhibitory factor to promote abnormal bone remodeling of subchondral bone in osteoarthritis. BMC Musculoskelet Disord. 2022;23:87.
Jin H-K, Park J-S, Kim J-M. The effect of microcurrent stimulation intensity on osteoarthritis in rat. Phys Therapy Korea. 2011;18:83–92.
Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, Morris EA, Glasson SS. Double-knockout of ADAMTS‐4 and ADAMTS‐5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheumatism: Official J Am Coll Rheumatol. 2007;56:3670–4.
Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J, Bian F, Chang B, Bai X, Han W. Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthr Cartil. 2018;26:1063–9.
Hong JH, Moon SM, Lee MJ, Kim CS. Efficacy of Aqueous Extract of Anthriscus sylvestris Leaves in Alleviating inflammation and Improving Articular Cartilage in an In Vivo Experimental Model. 2022.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C2093828) and "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005).
Author information
Authors and Affiliations
Contributions
M. Lee, H. Lee, and H.S. Kim contributed to the study conception, data collection, and analysis. The first draft was written by M. Lee and H. Lee and all authors edited and supervised the revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocols for all procedures were approved by the Yonsei University Animal Care Committee (YWCI-202102-002-01).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M., Lee, H., Chung, H. et al. Micro-current stimulation could inhibit IL-1β-induced inflammatory responses in chondrocytes and protect knee bone cartilage from osteoarthritis. Biomed. Eng. Lett. (2024). https://doi.org/10.1007/s13534-024-00376-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13534-024-00376-1