Abstract
Purpose: Severe side effects prevent the utilization of otherwise promising drugs in treatments. These side effects arise when drugs affect untargeted tissues due to poor target specificity. In photopharmacology, light controls the timing and the location of drug delivery, improving treatment specificity and pharmacokinetic control. Photopharmaceuticals have not seen widespread adoption in part because researchers do not always have access to reliable and reproducible light delivery devices at prices which fit within the larger research budget. Method: In this work, we present a customizable photomodulator for use in both wearable and implantable devices. For experimental validation of the photomodulator, we photolyse JF-NP-26 in rats. Results: We successfully drive in vivo photopharmacology with a tethered photomodulator and demonstrate modifications which enable the photomodulator to operate wirelessly. Conclusion: By documenting our photomodulator development, we hope to introduce researchers to a simple solution which significantly lowers the engineering barriers to photopharmacology research.
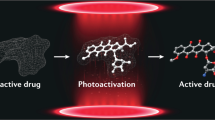
Graphical abstract
Researchers present a photomodulator, a device designed to facilitate in vivo photopharmacology. They demonstrate the in vivo capabilities of the photomodulator by photoreleasing raseglurant, an mGluR5 inhibitor, to treat pain in an acute rat model and follow this study by showing how to reconfigure the photomodulator to work wirelessly and interface with other biomedical devices.








Similar content being viewed by others
References
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. https://doi.org/10.1038/natrevmats.2016.71.
Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–56. https://doi.org/10.1016/j.addr.2016.02.006.
Tietze R, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468(3):463–70. https://doi.org/10.1016/j.bbrc.2015.08.022.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. https://doi.org/10.1038/nbt.3330.
Hüll K, Morstein J, Trauner D. In Vivo Photopharmacology. Chem Rev. 2018;118(21):10710–47. https://doi.org/10.1021/acs.chemrev.8b00037.
Pittolo S, et al. An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat Chem Biol. 2014;10(10):813–5. https://doi.org/10.1038/nchembio.1612.
Tibbitt MW, Dahlman JE, Langer R. Emerging frontiers in drug delivery. J Am Chem Soc. 2016;138(3):704–17. https://doi.org/10.1021/jacs.5b09974.
Dcona MM, Sheldon JE, Mitra D, Hartman MCT. Light induced drug release from a folic acid-drug conjugate. Bioorg Med Chem Lett. 2017;27(3):466–9. https://doi.org/10.1016/j.bmcl.2016.12.036.
Nani RR, Gorka AP, Nagaya T, Kobayashi H, Schnermann MJ. Near-IR light-mediated cleavage of antibody-drug conjugates using cyanine photocages. Angew Chem Int Ed. 2015;54(46):13635–8. https://doi.org/10.1002/anie.201507391.
Izquierdo-Serra M, et al. Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches. Nat Commun. 2016;7(1):12221. https://doi.org/10.1038/ncomms12221.
Paoletti P, Ellis-Davies GCR, Mourot A. Optical control of neuronal ion channels and receptors. Nat Rev Neurosci. 2019;20(9):514–32. https://doi.org/10.1038/s41583-019-0197-2.
Font J, et al. “Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife. 2017;6:e23545. https://doi.org/10.7554/eLife.23545.
Anikeeva P, et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2012;15(1):163–70. https://doi.org/10.1038/nn.2992.
Wang J, et al. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J Neural Eng. 2012;9(1): 016001. https://doi.org/10.1088/1741-2560/9/1/016001.
Kim T, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340(6129):211–6. https://doi.org/10.1126/science.1232437.
Jeong J-W, et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell. 2015;162(3):662–74. https://doi.org/10.1016/j.cell.2015.06.058.
Lee ST, Williams PA, Braine CE, Lin D-T, John SWM, Irazoqui PP. A miniature, fiber-coupled, wireless, deep-brain optogenetic stimulator. IEEE Trans Neural Syst Rehabil Eng. 2015;23(4):655–64. https://doi.org/10.1109/TNSRE.2015.2391282.
M. Schwaerzle, P. Ringwald, O. Paul, and P. Ruther, (2017) “First dual-color optrode with bare laser diode chips directly butt-coupled to hybrid-polymer waveguides,” in 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA: IEEE, pp. 526–529. doi: https://doi.org/10.1109/MEMSYS.2017.7863459.
Shin G, et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron. 2017;93(3):509-521.e3. https://doi.org/10.1016/j.neuron.2016.12.031.
Qazi R, et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat Biomed Eng. 2019;3(8):655–69. https://doi.org/10.1038/s41551-019-0432-1.
Zhang Y, et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc Natl Acad Sci USA. 2019;116(43):21427–37. https://doi.org/10.1073/pnas.1909850116.
Zhang Y, et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci Adv. 2019;5(7):eaaw5296. https://doi.org/10.1126/sciadv.aaw5296.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Pr; 1982.
Gong N, Huang Q, Chen Y, Xu M, Ma S, Wang Y-X. Pain assessment using the rat and mouse formalin tests. Bio-Protoc. 2014. https://doi.org/10.21769/BioProtoc.1288.
Bhamra H, Tsai J-W, Huang Y-W, Yuan Q, Shah JV, Irazoqui P. A Subcubic millimeter wireless implantable intraocular pressure monitor microsystem. IEEE Trans Biomed Circuits Syst. 2017;11(6):1204–15. https://doi.org/10.1109/TBCAS.2017.2755596.
Pederson DJ, et al. The bionode: a closed-loop neuromodulation implant. ACM Trans Embed Comput Syst. 2019;18(1):1–20. https://doi.org/10.1145/3301310.
Ottaviani MM, Vallone F, Micera S, Recchia FA. Closed-loop vagus nerve stimulation for the treatment of cardiovascular diseases: state of the art and future directions. Front Cardiovasc Med. 2022;9: 866957. https://doi.org/10.3389/fcvm.2022.866957.
Newman JP, Fong M, Millard DC, Whitmire CJ, Stanley GB, Potter SM. Optogenetic feedback control of neural activity. Elife. 2015;4:e07192. https://doi.org/10.7554/eLife.07192.
A. Sinicropi, “BIOMIMETIC PHOTOSWITCHES,” La Chimica & L’Industria, p. 8, Apr. 2010.
Babii O, et al. “Peptide drugs for photopharmacology: how much of a safety advantage can be gained by photocontrol? Future Drug Discovery. 2020;2(1):FDD28. https://doi.org/10.4155/fdd-2019-0033.
Sansalone L, Bratsch-Prince J, Tang S, Captain B, Mott DD, Raymo FM. Photopotentiation of the GABA A receptor with caged diazepam. Proc Natl Acad Sci USA. 2019;116(42):21176–84. https://doi.org/10.1073/pnas.1902383116.
Trads JB, et al. Sign inversion in photopharmacology: incorporation of cyclic azobenzenes in photoswitchable potassium channel blockers and openers. Angew Chem Int Ed. 2019;58(43):15421–8. https://doi.org/10.1002/anie.201905790.
Acknowledgements
The authors thank Yvonne Chen, Brett Collar, and Trevor Meyer, for fabrication and procedural assistance and Ryan Budde, Brett Collar, and Dr. Jay Shah for providing manuscript feedback. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animals were performed in accordance with the ethical standards of the institution or practice at which the studies were conducted (PACUC Protocol #1807001777).
Funding
This research was funded by Eli Lilly and Company (Lilly) as part of the Connected Solutions research initiative between Lilly and Purdue University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Ajieren and P. Irazoqui have received royalties as part of a licensing agreement with Eli Lilly and are inventors on a patent application submitted by Eli Lilly for technology related to this research.
Ethical approval
All procedures involving animals occurred under protocol number 1807001777 as approved by the Institutional Animal Care and Use Committee of Purdue University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajieren, H., Fox, A., Biggs, E. et al. Design and validation of a low-cost photomodulator for in vivo photoactivation of a mGluR5 inhibitor. Biomed. Eng. Lett. 14, 245–254 (2024). https://doi.org/10.1007/s13534-023-00334-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-023-00334-3