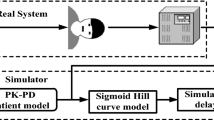
Abstract
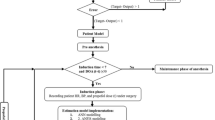
In the anesthesia automation, an automatic propofol infusion system uses Bi-spectral Index Signal (BIS) as a primary feedback signal to manipulate propofol dose. However, the BIS signal may be suspended for some time due to poor EEG signal quality, noise, and many other factors. Therefore, BIS signal failure may be the main cause of inadequate propofol infusion. This fact motivates the need for integration of multivariable fault tolerance module (MFTM) and fractional-order Smith predictor controller to avoid adverse reactions of inadequate propofol dosing during BIS failure. Smith Predictor control strategy is sufficiently robust to predict feedback BIS during BIS failure via patient pharmacological modeled BIS. However, modeled BIS may not provide a guarantee of adequate propofol infusion during BIS failure and especially in the presence of hypotension and hypertension. Thus, the proposed control strategy is designed with MFTM to detect BIS sensor fault and to estimate feedback BIS during BIS failure. Further, the proposed control strategy is designed with a multivariable pharmacological patient model to analyze the cross effect of propofol infusion on BIS and hemodynamic variables. The robustness of the proposed control strategy is tested in the presence of noxious surgical stimulation, BIS sensor fault and heavy hemodynamic disturbance. The pharmacological parameters and recorded signals of 30 patients during various surgeries have been used to validate simulated results. The performance of the proposed control strategy assures optimization and smooth propofol infusion during BIS failure. The proposed system provides stability for a wide range of physiological parameters range. The proposed scheme maintains smooth BIS and MAP signal despite the delay, BIS sensor fault, and surgical disturbances.













Similar content being viewed by others
References
Struys MM, De Smet T, Versichelen LF, Van De Velde S, Van den Broecke R, Mortier EP. Comparison of closed-loop controlled administration of propofol using bispectral Index as the controlled variable versus standard practice controlled administration. Anesthesiology. 2001;95(1):6–17.
Ilyas M, Butt MFU, Bilal M, Mahmood K, Khaqan A, Riaz, RA. A review of modern control strategies for clinical evaluation of propofol anesthesia administration employing hypnosis level regulation. Hindawi BioMed Research International, 2017;2017:1–12.
Neckebroek M, De Smet T, Struys M. Automated drug delivery in anesthesia. Curr Anesthesiol Rep. 2013;3:18–26.
Absalom A, Sutcliffe N, Kenny G. Closed-loop control of anesthesia using Bi spectral index: performance assessment in patients undergoing major orthopedic surgery under combined general and regional anesthesia. Anesthesiology. 2002;96:67–73.
Reboso JA, Méndez JA, Reboso HJ, León AM. Design and implementation of a closed-loop control system for infusion of propofol guided by bispectral index (BIS). Acta Anaesthesiol Scand. 2012;56(8):1032–41.
Heusden KV, Dumont GA, Soltesz K, et al. Design and clinical evaluation of robust PID control of propofol anesthesia in children. IEEE Trans Control Syst Technol. 2014;22(2):491–501.
Padula F, Ionescu C, Latronico N, et al. Optimized PID control of depth of hypnosis in anesthesia. Comput Methods Programs Biomed. 2017;144:21–35.
Merigo L, Beschi M, Padula F, Latronico N, Paltenghi M, Visioli A. Event-based control of depth of hypnosis in anesthesia. Comput Methods Programs Biomed. 2017;147:63–83.
Yoshihito S, Eiko F, Gotaro S, Mituhiko A, Kazuhiko F. A model predictive hypnosis control system under total intravenous anesthesia. IEEE Trans Biomed Eng. 2008;55(3):874–87.
Naşcu I, Krieger A, Ionescu CM, Pistikopoulos EN. Advanced model-based control studies for the induction and maintenance of intravenous anesthesia. IEEE Trans Biomed Eng. 2015;62(3):832–41.
Ionescu C, Machado JT, De Keyser R, Decruyenaere J, Struys M. Nonlinear dynamics of the patient’s response to drug effect during general anesthesia. Commun Nonlinear Sci Numer Simul. 2015;20:914–26.
Ionescu CM, Copot D, Keyser R. Anesthesiologist in the loop and predictive algorithm to maintain hypnosis while mimicking surgical disturbance. IFAC Pap Online. 2017;50(1):15080–5.
Neckebroek M, Ionescu CM, van Amsterdam K, De Smet T, De Baets P, Decruyenaere J, De Keyser R, Struys MMRF. A comparison of propofol-to-BIS post-operative intensive care sedation by means of target-controlled infusion, Bayesian-based and predictive control methods: an observational open-label pilot study. J Clin Monit Comput. 2019;33(4):675–686.
Ilyas M, Iqbal J, Ahmad S, et al. Hypnosis regulation in propofol anesthesia employing super-twisting sliding mode control to compensate variability dynamics. IET Syst Biol. 2020;14(2):59–67.
Ilyas M, Khaqana A, Iqbalb J, Riaz RA. Regulation of hypnosis in propofol anesthesia administration based on non-linear control strategy. Braz J Anesthesiol. 2017;67(2):122–30.
Yelneedi S, Samavedham L, Rangaiah GP. A comparative study of three advanced controllers for the regulation of hypnosis. J Process Control. 2009;19(9):1458–69.
Méndez JA, Torres S, Reboso JA, Reboso H. Adaptive computer control of anesthesia in humans. Comput Methods Biomech Biomed Eng. 2009;12(6):727–34.
Bhavina P, Hiren P, Pragna V, Divyang S, Alpesh S. Adaptive Smith predictor controller for total intravenous anesthesia automation. Biomed Eng Lett. 2019;9(1):127–44.
Abdulla SA, Wen P. Robust internal model control for depth of anesthesia. Int J Mechatron Autom. 2011;1(1):1–8.
Mendez JA, Leon A, Marrero A, Gonzalez-Cava JM, Reboso JA, Estevez JI, Gomez-Gonzalez JF. Improving the anesthetic process by a fuzzy rule based medical decision system. Artif Intell Med. 2018;84:159–70.
Padmanabhan R, Meskin N, Haddad WM. Closed-loop control of anesthesia and mean arterial pressure using reinforcement learning. Biomed Signal Process Control. 2015;22:54–64.
Dumont GA, Martinez A, Ansermino JM. Robust control of depth of anesthesia. Int J Adapt Control Signal Process. 2009;23:435–54.
Ajwad SA, Iqbal J, Ullah MI, et al. A systematic review of current and emergent manipulator control approaches. Front Mech Eng. 2015;10:198–210.
Iqbal J, et al. Nonlinear control systems—a brief overview of historical and recent advances. Nonlinear Eng. 2017;6(4):301–12.
Ullah S, Mehmood A, Khan Q, et al. Robust integral sliding mode control design for stability enhancement of under-actuated quadcopter. Int J Control Autom Syst. 2020;18:1671–8.
Jamshed Iqbal. Modern control laws for an articulated robotic arm: modeling and simulation engineering. Technol Appl Sci Res. 2019;9(2):4057–61.
Ionescu CM, Lopes A, Tenreiro Machado J, Bates J. The role of fractional calculus in modeling biological phenomena: a review. Commun Nonlinear Sci Numer Simul. 2017;51:141–59.
Muresan CI, Dutta A, Dulf EH, Pinar Z, Maxim A, Ionescu CM. Tuning algorithms for fractional order internal model controllers for time delay processes. Int J Control. 2016;89(3):579–93.
De Keyser R, Muresan CI, Ionescu C. A novel auto-tuning method for fractional order PI/PD controllers. ISA Trans. 2016;62:268–75.
De Keyser R, Ionescu CM, Muresan CI. Comparative evaluation of a novel principle for PID auto tuning. In: 11th the Asian control conference gold coast convention Centre Australia, 2017; p. 1164–9.
Muresan CI, Birs IR, Prodan O, Nascu I, De Keyser R. Approximation methods for FO-IMC controllers for time delay systems. In: E3S web of conferences, 2019; p. 115.
Birs IR, Muresan CI, Nascu I, Ionescu CM. A survey of recent advances in fractional order control for time delay systems. IEEE Access. 2019;7:30951–65.
Marzieh S, Saeed T. Smith predictor based fractional-order control design for time-delay integer-order systems. Int J Dyn Control. 2017;6(1):180–7.
Castillo-Garcia FJ, Feliu-Batlle V, Rivas-Perez R. Time domain tuning of fractional order controllers combined with a Smith predictor for automation of water distribution in irrigation main channel pools. Asian J Control. 2013;15:819–33.
Ionescu CM. A computationally efficient Hill curve adaptation strategy during continuous monitoring of dose–effect relation in anaesthesia. Nonlinear Dyn. 2018;92(3):843–52.
Fan SZ, Wei Q, Shi PF, Chen YJ, Liu Q, Shieh JS. A comparison of patient’s heart rate variability and blood flow variability during surgery based on the Hilbert Huang transform. Biomed Signal Process Control. 2012;7(5):465–73.
Frei CW. Fault tolerant control concepts applied to anesthesia. Ph.D. thesis, ETH Zurich; 2000.
Yu Y-N, Doctor F, Fan S-Z, Shieh J-S. An adaptive monitoring scheme for automatic control of anesthesia in dynamic surgical environments based on bispectral index and blood pressure. J Med Syst. 2018;42(5):95.
Sang WL, Soo EC, Jin Hee H, Sung-Wook P, Wha Ja K, Young KC. Effect of beach chair position on Bi spectral index values during arthroscopic shoulder surgery. Korean J Anesthesiol. 2014;67(4):235–9.
Tao Y, Fang M, Wang Y. A fault tolerant closed loop anesthesia system based on internal model control and extended state observer. In: 25th Chinese control and decision conference (CCDC), 2013; p. 4910–4.
Neckebroek M, Boldingh JW, De Smet T, Struys MM. Influence of remifentanil on the control performance of the bispectral index controlled Bayesian-based closed-loop system for propofol administration. Anesth Analg. 2020;130(6):1661–9.
Jeleazcov C, Lavielle M, Schüttler J, Ihmsen H. Pharmacodynamics response modeling of arterial blood pressure in adult volunteers during propofol anesthesia. Br J Anesth. 2015;115(2):213–26.
Ionescu CM, Hodrea R, Keyser R. Variable time-delay estimation for anesthesia control during intensive care. IEEE Trans Biomed Eng. 2011;58(2):363–9.
Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92(3):727–38.
Martín-Mateos I, Pérez JM, Morales JR, Gómez-González JF. Adaptive pharmacokinetic and pharmacodynamics modeling to predict propofol effect using BIS-guided anesthesia. Comput Biol Med. 2016;75:173–80.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Bonate PL. Pharmacokinetic-pharmacodynamics modeling and simulation. 2nd ed. New York: Springer; 2011.
Birs I, Copot D, Muresan CI, De Keyser R, Ionescu CM. Robust fractional order PI control for cardiac output stabilization. IFAC Papers Online, Florianópolis - SC, Brazil, 2019:52(1):994-99.
Bode HW. Network analysis and feedback amplifier design. New York: Van Nostrand; 1945.
Chevalier A, Francis C, Copot C, Ionescu CM, De Keyser R. Fractional-order PID design: towards transition from state-of-art to state-of-use. ISA Trans. 2018. https://doi.org/10.1016/j.isatra.2018.09.017.
Struys MM, Smet T, Greenwald S, Absalom AR, Bing S, Mortier EP. Performance evaluation of two published closed-loop control systems using bi spectral index monitoring: a simulation study. Anesthesiology. 2004;100(3):640–7.
Acknowledgements
The authors would like to thank to anesthesia department team of the SMIMER hospital, Surat for providing the clinical environment facility and drug dose combination as per proposed scheme. The authors are grateful to anonymous reviewers for their useful suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the Surat Municipal Institute of Medical Education and Research (SMIMER), India and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables 8, 9, 10, 11, 12 and Figs. 10 and 11.
Rights and permissions
About this article
Cite this article
Patel, B., Patel, H., Shah, D. et al. Control strategy with multivariable fault tolerance module for automatic intravenous anesthesia. Biomed. Eng. Lett. 10, 555–578 (2020). https://doi.org/10.1007/s13534-020-00169-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00169-2