Abstract
Purpose
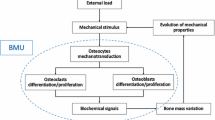
Optimal bone remodeling is responsible for bone health and strength, and an imbalance in this process may cause diseases such as osteoporosis. Both osteocyte density and mechanosensitivity are effective parameters in changing bone apparent density. This study aimed at investigating the effects of osteocyte density in healthy adults, and osteocyte mechanosensitivity in osteoporotic bones, on bone loss using a semi-mechanistic bone remodeling theory.
Methods
A 2-D finite element model of trabecular bone was developed in order to simulate the process of bone remodeling. The structure was loaded by a sinusoidal stress, cycling between 0 and 2 MPa, and at frequency of 1 Hz. It was assumed that the stimulus sensed by osteocytes is the maximal strain energy density rate. By changing osteocyte density and mechanosensitivity, the effects of altering external load magnitude and/or direction were investigated.
Results
First, trabecular-like structures were obtained from the initial configuration, in which trabeculae were lined up with the loading direction as verification of model’s implementation. Secondly, it was demonstrated that bone loss could occur in healthy older trabecular bone model, compared to healthy young bone model. Finally, this in silico study showed that by decreasing osteocyte mechanosensitivity, assuming a constant osteocyte density, a decrease in bone apparent density is predicted.
Conclusions
Results of this work indicated that the semimechanistic model used here may reasonably explain bone loss in healthy adults and in osteoporotic patients. Moreover, this study sheds more light on the possible effects that osteocyte density in healthy adults, and osteocyte mechanosensitivity in osteoporotic individuals, may have on bone apparent density predictions.
Similar content being viewed by others
References
Ruimerman R, Huiskes R. Development of a unifying theory for mechanical adaptation and maintenance of trabecular bone. Theor Issues Ergon Sci. 2005; 6(3):4–225.
Ruimerman R, Hilbers P, van Rietbergen B, Huiskes R. A theoretical framework for strain-related trabecular bone maintenance and adaptation. J Biomech. 2005; 38(4):931–41.
Rouhi G. Biomechanics of Osteoporosis: The Importance of Bone Resorption and Remodeling Processes. In: Dionyssiotis Y, editors. Osteoporosis. Rijeka: Intech; 2012. pp. 59–80.
Rouhi G, Epstein M, Sudak, L, Herzog W. Modeling bone resorption using mixture theory with chemical reactions. J Mech Mater Struct. 2007; 2(6):1141–55.
Rouhi G. A tri-phasic mixture model of bone resorption: theoretical investigations. J Mech Behav Biomed Mater. 2011; 4(8):1947–54.
Wolff J. The law of bone remodelling. 1st ed. Berlin: Springer; 1986.
Frost HM. Mathematical elements of lamellar bone remodelling. 1st ed. Springfield: Charles C Thomas Pub Ltd; 1964.
Frost HM. A determinant of bone architecture: the minimum effective strain. Clin Orthop Relat Res. 1983; 175:286–92.
Cowin CS, Hegedus DH. Bone remodeling I: a theory of adaptive elasticity. J Elast. 1976; 6(3):313–26.
Hegedus DH, Cowin CS. Bone remodeling II: small strain adaptive elasticity. J Elast. 1976; 6(4):337–52.
Rouhi G, Herzog W, Sudak L, Firoozbakhsh K, Epstein M. Free surface density instead of volume fraction in the bone remodeling equation: theoretical considerations. Forma. 2004; 19(3):165–82.
Rouhi G, Epstein M, Sudak L, Herzog W. Free surface density and microdamage in the bone remodeling equation: theoretical considerations. Int J Eng Sci. 2006; 44(7):456–69.
Huiskes, R, Ruimerman R, van Lenthe GH, Jassen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000; 405(6787):704–6.
Vahdati A, Rouhi G. A model for mechanical adaptation of trabecular bone incorporating cellular accommodation and effects of microdamage and disuse. Mech Res Commun. 2009; 36(3):284–93.
Rouhi G, Vahdati A, Li X, Sudak L. A three dimensional computer model to simulate trabecular bone remodeling under overload using a semi-mechanistic bone remodeling theory. J Mech Med Biol. 2015; 15(4):1550061(1-18);doi:10.1142/S021951941550061X.
Mullender MG, van der Meer DD, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996; 18(2):109–13.
Mori S, Harruff R, Ambrosius W, Burr DB. Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. Bone. 1997; 21(6):521–6.
Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000; 26(4):375–80.
Parfitt AM. Life history of osteocytes: relationship to bone age, bone remodeling, and bone fragility. J Musculoskelet Neuronal Interact. 2002; 2(6):499–500.
Klein-Nulend J, Sterck JG, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH. Donor age and mechanosensitivity of human bone cells. Osteoporos Int. 2002; 13(2):137–46.
Vashishth D, Gibson GJ, Fyhrie DP. Sexual dimorphism and age dependence of osteocyte lacunar density for human vertebral cancellous bone. Anat Rec A Discov Mol Cell Evol Biol. 2005; 282(2):157–62.
Cho S, Eom S, Seo D-H, Park J, Ko C-Y, Kim HS. Enhancement of bone quality and longitudinal growth due to free-fall motion in growing rats. Biomed Eng Lett. 2015; 5(2):73–8.
Rodan GA. Mechanical loading, estrogen deficiency, and the coupling of bone formation to bone resorption. J Bone Miner Res 1991; 6(6):527–30.
Currey JD. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech 1988; 21(2):131–9.
Ruimerman R, Huiskes R, van Lenthe GH, Janssen JD. A computer simulation model relating bone-cell metabolism to mechanical adaptation of trabecular architecture. Comput Methods Biomech Biomed Eng. 2001; 4(5):433–48.
Mullender MG, Huiskes R. A proposal for the regulatory mechanism of Wolff’s law. J Orthop Res. 1995; 13(4):503–12.
Baiotto S, Zidi M. Theoretical and numerical study of a bone remodeling model: The effect of osteocyte cells distribution. Biomech Model Mechnobiol. 2004; 3(1):6–16.
Marotti G, Remaggi F, Zaffe D. Quantitative investigation on osteocyte canaliculi in human compact and trabecular bone. Bone. 1985; 6(5):335–7.
Rüegsegger P. Imaging of bone structure. In: Cowin SC, Editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001, chapter 9, pp 1–24.
Sterck JG, Klein-Nulend J, Lips P, Burger EH. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am J Physiol. 1998; 274(6 Pt 1):E1113-20.
Mulvihill BM, Prendergast PJ. An algorithm for bone mechanoresponsiveness: implementation to study the effect of patient-specific cell mechanosensitivity on trabecular bone loss. Comput Methods Biomech Biomed Eng. 2008; 11(5):443–51.
Eriksen EF, Kassem M. The cellular basis of bone remodeling. Triangle. 1992; 31(2):45–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouhi, G., Vahdati, A., Li, X. et al. An investigation into the effects of osteocytes density and mechanosensitivity on trabecular bone loss in aging and osteoporotic individuals. Biomed. Eng. Lett. 5, 302–310 (2015). https://doi.org/10.1007/s13534-015-0206-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-015-0206-y