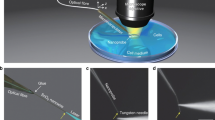
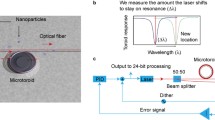
Abstract
Although recent advances in biosensing are promising, there still is a need for label-free, sensitive, and cost-effective detection of single nanoparticles and single biomolecules, without relying in complex fabrications and sophisticated detection strategies. In this review, we show that a better understanding of light-matter interactions can lead to the development of advanced biosensing and bioanalysis systems. In particular, we address alternative biosensing methods of taking advantage of naturally occurring lasers (also known as random lasers). In random lasers, resonances are self-formed due to multiple scattering, leading to light amplification and coherent light generation in the presence of amplifying media. In this case, lasing emission can be unprecedentedly sensitive to subtle nanoscale perturbations. Specifically, we discuss the possibility that random lasers can be an alternative yet superior physical mechanism for biosensors, because the random laser biosensing platform is simple and the detection strategy is straightforward. We further envision that random laser biosensors have the potential to facilitate developing enhanced biological and chemical sensors for single-molecule and single-nanoparticle quantitation.
Similar content being viewed by others
References
Sako Y, Minoghchi S, Yanagida T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nat Cell Biol. 2000; 2(3):168–72.
Jablonski AE, Humphries WH, Payne CK. Pyrenebutyratemediated delivery of quantum dots across the plasma membrane of living cells. J Phys Chem B. 2009; 113(2):405–8.
Lipman EA, Schuler B, Bakajin O, A. EW. Single-molecule measurement of protein folding kinetics. Science. 2003; 301(5637):1233–5.
Lang MJ, Fordyce PM, Engh AM, Neuman KC, Block SM. Simultaneous, coincident optical trapping and single-molecule fluorescence. Nat Methods. 2004; 1(2):133–39.
Lavrinenko AV, Wohlleben W, Leyrer RJ. Influence of imperfections on the photonic insulating and guiding properties of finite Si-inverted opal crystals. Opt Express. 2009; 17(2):747–60.
Choi SH, Kwak B, Han B, Kim YL. Competition between excitation and emission enhancements of quantum dots on disordered plasmonic nanostructures. Opt Express. 2012; 20(15):16785–93.
Huang B, Wu H, Bhaya D, Grossman A, Granier S, Kobilka BK, Zare RN. Counting low-copy number proteins in a single cell. Science. 2007; 315(5808):81–4.
Elf J, Li GW, Xie XS. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007; 316(5828):1191–4.
Moore BD, Stevenson L, Watt A, Flitsch S, Turner NJ, Cassidy C, Graham D. Rapid and ultra-sensitive determination of enzyme activities using surface-enhanced resonance Raman scattering. Nat Biotechnol. 2004; 22(9):1133–8.
Kulkarni RP, Castelino K, Majumdar A, Fraser SE. Intracellular transport dynamics of endosomes containing DNA polyplexes along the microtubule network. Biophys J. 2006; 90(5):L42–4.
Funatsu T, Harada Y, Tokunaga M, Saito K, Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995; 374(6522):555–9.
Myong S, Rasnik I, Joo C, Lohman TM, Ha T. Repetitive shuttling of a motor protein on DNA. Nature. 2005; 437(7063):1321–5.
Cao YWC, R. J, A. MC. Nanoparticles with raman spectroscopic fingerprints for dna and rna detection. Science. 2002; 297(5586):1536–40.
Hughes RC, Ricco AJ, Butler MA, Martin SJ. Chemical microsensors. Science. 1991; 254(5028):74–80.
Copper MA. Optical biosensors in drug discovery. Nat Rev Drug Discov. 2002; 1(7):515–28.
Choi SH, Kim YL, Byun KM. Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt Express. 2011; 19(2):458–66.
Choi SH, Byun KM. Investigation on an application of silver substrates for a sensitive surface plasmon resonance imaging detection. J Optl Soc of Am A. 2010; 27(10):2229–36.
Choi SH, Kim SJ, Byun KM. Characteristics of light emission from surface plasmons based on rectangular silver gratings. Opt Commun. 2010; 283(14):2961–6.
Choi SH, Kim SJ, Byun KM. Design study for transmission improvement of resonant surface plasmons using dielectric diffraction gratings. Appl Opt. 2009; 48(15):2924–31.
Piehler J, A. B, G. G. Affinity detection of low molecular weight analyte. Anal Chem. 1996; 68(1):139–43.
Cross GH, Reeves A, Brand S, Swann MJ, Peel LL, Freeman NJ, Lu JR. The metrics of surface adsorbed small molecules on the Young’s fringe dual-slab waveguide interferometer. J Phys D Appl Phys. 2004; 37(1):74–80.
Choi CJ, Block ID, Bole B, Dralle D, Cunningham BT. Labelfree photonic crystal biosensor integrated microfluidic chip for determination of kinetic reaction rate constants. IEEE Sens J. 2009; 9(12):1697–704.
Cunningham BT, Li P, Schulz S, Lin B, Baird C, Gerstenmaier J, Genick C, Wang F, Fine E, Laing L. Label-free assays on the bind system. J Biomol Screen. 2004; 9(6):481–90.
Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003; 301(5641):1884–6.
Zhong Z, Wang D, Cui Y, Bockrath MW, Lieber CM. Nanowire crossbar arrays as address decoders for integrated nanosystems. Science. 2003; 302:(5649):1377–9.
Burg TP, Godin M, Knudsen SM, Shen W, Carlson G, Foster JS, Babcock K, Manalis SR. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature. 2007; 446(7139):1066–9.
Yeo WS, Min DH, Hsieh RW, Greene GL, Mrksich M. Labelfree detection of protein-protein interactions on biochips. Angew Chem Int Edit. 2005; 44(34):5480–3.
Armani AM, Kulkarni RP, Fraser SE, Flagan RC, Vahala KJ. Label-free, single-molecule detection with optical microcavities. Science. 2007; 317(5839):783–7.
Vollmer F, Arnold S. Whispering-gallery-mode biosensing: label-free detection down to single molecules. Nat Methods. 2008; 5(7):591–6.
Harker A, Mehrabani S, Armani AM. Ultraviolet light detection using an optical microcavity. Opt Lett. 2013; 38(17):3422–5.
Zhu J, Ozdemir SK, Xiao YF, Li L, He L, Chen DR, Yang L. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonator. Nat Photonics. 2010; 4(1):46–9.
Dominguez-Juarez JL, Kozyreff G, Martorell J. Whispering gallery microresonators for second harmonic light generation from a low number of small molecules. Nat Commun. 2011; doi:10.1038/ncomms1253.
Koenderink AF, Lagendijk A, Vos WL. Optical extinction due to intrinsic structural variations of photonic crystals. Phys Rev B. 2005; doi:10.1103/PhysRevB.72.15312.
Toninelli C, Vekris E, Ozin GA, John S, Wiersma DS. Exceptional reduction of the diffusion constant in partially disordered photonic crystals. Phys Rev Lett. 2008; doi:10.1103/PhysRevLett.101.12391.
Woldering LA, Mosk AP, Tjerkstra RW, Vos WL. The influence of fabrication deviations on the photonic band gap of three-dimensional inverse woodpile nanostructures. J Appl Phys. 2009; doi:10.1063/1.310377.
Lavrinenko AV, Wohlleben W, Leyrer RJ. Influence of imperfections on the photonic insulating and guiding properties of finite Si-inverted opal crystals. Opt Express. 2009; 17(2):747–60.
Wiersma DS. Disordered photonics. Nat Photonics 2013; 7(3):188–96.
Cao H. Review on latest developments in random lasers with coherent feedback. J Phys A-Math Gen. 2005; 38(49):10497–535.
Noginov M. Solid-State Random Lasers. 1st ed. Berlin: Springer; 2005.
Wiersma DS. The physics and applications of random lasers. Nat Phys. 2008; 4(5):359–67.
Nishijima Y, Ueno K, Juodkazis S, Mizeikis V, Fujiwara H, Sasaki K, Misawa H. Lasing with well-defined cavity modes in dye-infiltrated silica inverse opals. Opt Express. 2009; 17(4):2976–83.
Wiersma DS, van Albada MP, van Tiggelen BA, Lagendijk A. Experimental evidence for recurrent multiple-scattering events of light in disordered media. Phys Rev Lett. 1995; 74(21):4193–6.
Polson RC, Chipouline A, Vardeny ZV. Random lasing in piconjugated films and infiltrated opals. Adv Mater. 2001; 13(10):760–4.
Kim YL, Turzhitsky VM, Liu Y, Roy HK, Wali RK, Subramanian H, Pradhan P, Backman V. Low-coherence enhanced backscattering: Review of principles and applications for colon cancer screening. J Biomed Opt. 2006; doi:10.1117/1.2236292.
Wang W, Wu N, Tian Y, Wang X, Niezrecki C, Chen J. Optical pressure/acoustic sensor with precise Fabry-Perot cavity length control using angle polished fiber. Opt Express. 2009; 17(19):16613–8.
Hill GC, Melamud R, Declercq FE, Davenport AA, Chan IH, Hartwell PG, Pruitt BL. SU-8 mems fabry-perot pressure sensor. Sensors Actuat a-Phys. 2007; 138(1):52–62.
Cunningham BT. Label-free optical biosensors: An introduction. New York: Cambridge University Press; 2009.
Kringlebotn J, Archambault J, Reekie L, Payne D. Er3+yb3+ codoped fiber distributed-feedback laser. Opt Lett. 1994; 19(24):2101–3.
Koo KP, Kersey AD. Bragg grating-based laser sensors systems with interferometric interrogation and wavelengthdivision multiplexing. J Lightwave Technol. 1995; 13(7):1243–9.
Mujumdar S, Ricci M, Torre R, Wiersma DS. Amplified extended modes in random lasers. Phys Rev Lett. 2004; doi:10.1103/PhysRevLett.93.053903.
Mujumdar S, Türck V, Torre R, Wiersma DS. Chaotic behavior of a random laser with static disorder. Phys Rev A. 2007; doi:10.1103/PhysRevA.76.033807.
Polson RC, Vardeny ZV. Random lasing in human tissues. Appl Phys Lett. 2004; 85(7):1289–91.
Polson RC, Vardeny ZV. Organic random lasers in the weak-scattering regime. Phys Rev B. 2005; doi:10.1103/PhysRevB.71.045205.
Tulek A, Polson RC, Vardeny ZV. Naturally occurring resonators in random lasing of ·š-conjugated polymer films. Nat Phys. 2010; 6(4):303–10.
Wiersma DS, Cavalieri S. Light emission: a temperaturetunable random laser. Nature. 2001; 414(6865):708–9.
Rose A, Zhu Z, Madigan CF, Swager TM, Buloviæ V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature. 2005; 434(7035):876–9.
Deng C, He Q, He C, Shi L, Cheng J, Lin T. Conjugated polymer-titania nanoparticle hybrid films: random lasing action and ultrasensitive detection of explosive vapors. J Phys Chem B. 2010; 114(13):4725–30.
Letokhov VS. Generation of light by a scattering medium with negative resonance absorption. Sov J Phys. 1968; 26(4):835–40.
Lawandy NM, Balachandran RM, Gomes ASL, Sauvain E. Laser action in strongly scattering media. Nature. 1994; 368(6470):436–8.
Pradhan P, Kumar N. Localization of light in coherently amplifying random-media. Phys Rev B. 1994; 50(13):9644–7.
Song Q, Xu Z, Choi SH, Sun X, Xiao S, Akkus O, Kim YL. Detection of nanoscale structural changes in bone using random lasers. Biomed Opt Express. 2010; 1(5):1401–7.
Popov O, Zilbershtein A, Davidov D. Random lasing from dye-gold nanoparticles in polymer films: enhanced gain at the surface-plasmon-resonance wavelength. Appl Phys Lett. 2006; doi:10.1063/1.2364857.
Dominguez CT, Maltez RL, dos Reis RMS, de Melo LSA, de Araújo CB, Gomes ASL. Dependence of random laser emission on silver nanoparticle density in PMMA films containing rhodamine 6G. J Opt Soc Am B. 2011; 28(5):1118–23.
Cao H, Zhao YG, Ho ST, Seelig EW, Wang QH, Chang RPH. Random laser action in semiconductor powder. Phys Rev Lett. 1999; 82(11):2278–81.
Vanneste C, Sebbah P, Cao H. Lasing with resonant feedback in weakly scattering random systems. Phys Rev Lett, 2007. doi:10.1103/PhysRevLett.98.1439–2.
Muskens OL, Diedenhofen SL, Kaas BC, Algra RE, Bakkers EP, Rivas JG, Lagendijk A. Large photonic strength of highly tunable resonant nanowire materials. Nano Lett. 2009; 9(3):930–4.
Meng X, Fujita K, Murai S, Matoba T, Tanaka K. Plasmonically controlled lasing resonance with metallicdielectric core-shell nanoparticles. Nano Lett. 2011; 11(3):1374–8.
Siddique M, Yang L, Wang QZ, Alfano RR. Mirrorless laser action from optically pumped dye-treated animal-tissues. Opt Commun. 1995; 117(5–6):475–9.
Wang L, Liu D, He N, Jacques SL, Thomsen SL. Biological laser action. Appl Opt. 1996; 35(10):1775–9.
Polson RC, Vardeny ZV. Random lasing in human tissues. Appl Phys Lett. 2004; 85(7):1289–91.
Song Q, Xiao S, Xu Z, Liu J, Sun X, Drachev V, Shalaev VM, Akkus O, Kim YL. Random lasing in bone tissue. Opt Lett. 2010; 35(9):1425–7.
Gather MC, Yun SH. Single-cell biological lasers. Nat Photonics. 2011; 5(7):406–10.
Jiang X, Soukoulis CM. Localized random lasing modes and a path for observing localization. Phys Rev E. 2002; doi:10.1103/PhysRevE.65.025601.
Jiang X, Soukoulis CM. Transmission and reflection studies of periodic and random systems with gain. Phys Rev B. 1999; 59(9):6159–66.
Choi K, Kim H, Lim Y, Kim S, Lee B. Analysis design and visualization of multiple surface plasmon resonance excitation using angular spectrum decomposition for a gaussian input beam. Opt Express. 2005; 13(22):8866–74.
Hecht E. Optics. 4th ed. Seoul: Addison Wesley Longman; 2002.
Vanneste C, Sebbah P. Selective excitation of localized modes in active random media. Phys Riv Lett. 2001; doi:10.1103/PhysRevLett.87.183903.
Andreasen J, Asatryan AA, Botten LC, Byrne MA, Cao H, Ge L, Labonté L, Sebbah P, Stone AD, Türeci HE, Vanneste C. Modes of random lasers. Adv Opt Photonics. 2011; 3(1):88–127.
Wu X, Fang W, Yamilov A, Chabanov AA, Asatryan AA, Botten LC, Cao H. Random lasing in weakly scattering systems. Phys Rev A. 2006; doi:10.1103/PhysRevA.74.053812.
Ge L, Tandy RJ, Stone AD, Türeci HE. Quantitative verification of ab initio self-consistent laser theory. Opt Express. 2008; 16(21):16895–902.
Wu XH, Yamilov A, Noh H, Cao H, Seelig EW, Chang RP. Random lasing in closely packed resonant scatterers. J Opt Soc Am B. 2004; 21(1):159–67.
Jin J. The finite element method in electromagnetics. 2nd ed. New York: John Wiley & Sons; 1993.
Becker EB, Carrey GF, Oden JT. Finite elements: An introduction. NewJersey: Prentice Hall; 1991.
Wu XH, Cao H. Statistics of random lasing modes in weakly scattering systems. Opt Lett. 2007; 32(21):3089–91.
Wu X, Cao H. Statistical studies of random-lasing modes and amplified spontaneous-emission spikes in weakly scattering systems. Phys Rev A. 2008; doi:10.1103/PhysRevA.77.013832.
Tseng S, Kim Y, Taflove A, Maitland D, Backman V, Walsh J. Simulation of enhanced backscattering of light by numerically solving Maxwell’s equations without heuristic approximations. Opt Express. 2005; 13(10):3666–72.
Fallert J, Dietz RJ, Sartor J, Schneider D, Klingshirn C, Kalt H. Co-existence of strongly and weakly localized random laser modes. Nat Photonics. 2009; 3(5):279–82.
Frolov SV, Vardeny ZV, Yoshino K, Zakhidov A, Baughman RH. Stimulated emission in high-gain organic media. Phys Rev B. 1999; doi:10.1103/PhysRevB.59.R5284.
Van der Molen KL, Tjerkstra RW, Mosk AP, Lagendijk A. Spatial extent of random laser modes. Phys Rev Lett. 2007; doi:10.1103/PhysRevLett.98.143901.
Sebbah P, Vanneste C. Random laser in the localized regime. Phys Rev B. 2002; doi:10.1103/PhysRevB.66.144202.
Song Q, Xiao S, Xu Z, Shalaev VM, Kim YL. Random laser spectroscopy for nanoscale perturbation sensing. Opt Lett. 2010; 35(15):2624–6.
Shahar R, Zaslansky P, Barak M, Friesem AA, Currey JD, Weiner S. Anisotropic poisson's ratio and compression modulus of cortical bone determined by speckle interferometry. J Biomech. 2007; 40(2):252–64.
Choi S, Kim YL. Random lasing mode alterations by singlenanoparticle perturbations. Appl Phys Lett. 2012; doi:10.1063/1.3675885.
Jolliffe IT. Principal Component Analysis. 2nd ed. Springer-Verlag; 19–6.
Yamilov A, Cao H. Highest-quality modes in disordered photonic crystals. Phys Rev A. 2004; doi:10.1103/PhysRevA.69.031803.
Kopp VI, Fan B, Vithana HK, Genack AZ. Low-threshold lasing at the edge of a photonic stop band in cholesteric liquid crystals. Opt Lett. 1998; 23(21):1707–9.
Milner V, Genack AZ. Photon localization laser: low-threshold lasing in a random amplifying layered medium via wave localization. Phys Rev Lett. 2005; doi:10.1103/PhysRevLett.94.073901.
Choi SH, Kim YL. Hybridized/coupled multiple resonances in nacre. Phys Rev B. 2014; doi:10.1103/PhysRevB.89.035115.
Dice GD, Mujumdar S, Elezzabi AY. Plasmonically enhanced diffusive and subdiffusive metal nanoparticle-dye random laser. Appl Phys Lett. 2005; doi:10.1063/1.1894590.
Meng X, Fujita K, Zong Y, Murai S, Tanaka K. Random lasers with coherent feedback from highly transparent polymer films embedded with silver nanoparticles. Appl Phys Lett. 2008; doi:10.1063/1.2912527.
Meng X, Fujita K, Murai S, Tanaka K. Coherent random lasers in weakly scattering polymer films containing silver nanoparticles. Phys Rev A. 2009; doi:10.1103/PhysRevA.79.053827.
Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett. 2006; doi:10.1103/PhysRevLett.96.113002.
Bharadwaj P, Novotny L. Spectral dependence of single molecule fluorescence enhancement. Opt Express. 2007; 15(21):14266–74.
Choi SH, Kwak B, Han B, Kim YL. Competition between excitation and emission enhancements of quantum dots on disordered plasmonic nanostructures. Opt Express. 2012; 20(15):16785–93.
Chen Y, Munechika K, Ginger DS. Dependence of fluorescence intensity on the spectral overlap between fluorophores and plasmon resonant single silver nanoparticles. Nano Lett. 2007; 7(3):690–6.
Zhang Y, Dragan A, Geddes CD. Wavelength dependence of metal-enhanced fluorescence. J Phys Chem C. 2009; 113(28):12095–100.
Arnold S, Keng D, Shopova SI, Holler S, Zurawsky W, Vollmer F. Whispering gallery mode carousel — a photonic mechanism for enhanced nanoparticle detection in biosensing. Opt Express. 2009; 17(8):6230–8.
Lin VS, Motesharei K, Dancil KP, Sailor MJ, Ghadiri MR. A porous silicon-based optical interferometric biosensor. Science. 1997; 278(5339):840–3.
Morgan H, Taylor DM. A surface plasmon resonance immunosensor based on the streptavidin-biotin complex. Biosens Bioekctron. 1992; 7(6):405–10.
Abel AP, Weller MG, Duveneck GL, Ehrat M, Widmer HM. Fiber-optic evanescent wave biosensor for the detection of oligonucleotides. Anal Chem. 1996; 68(17):2905–12.
Rehák M, Šnejdárková M, Otto M. Application of biotinstreptavidin technology in developing a xanthine biosensor based on a self-assembled phospholipid membrane. Biosens Bioelectron. 1994; 9(4):337–41.
Kim DS, Park HJ, Park JE, Shin JK, Kang SW, Seo HI, Lim G. Mosfet-type biosensor for detection of streptavidin-biotin protein complexes. Sensors Mater. 2005; 17(5):259–68.
Jung YK, Park HG, Kim JM. Polydiacetylene (PDA)-based colorimetric detection of biotin-streptavidin interactions. Biosens Bioelectron. 2006; 21(8):1536–44.
Tao SL, Popat KC, Norman JJ, Desai TA. Surface modification of SU-8 for enhanced biofunctionality and nonfouling properties. Langmuir. 2008; 24(6):2631–6.
Sadana A, Ram AB. Fractal analysis of antigen-antibody binding kinetics: biosensor applications. Blotechnol Progr. 1994; 10(3):291–8.
Olkhov RV, Shaw AM. Label-free antibody-antigen binding detection by optical sensor array based on surface-synthesized gold nanoparticles. Biosens Bioelectron. 2008; 23(8):1298–302.
Nogues C, Leh H, Langendorf CG, Law RH, Buckle AM, Buckle M. Characterisation of peptide microarrays for studying antibody-antigen binding using surface plasmon resonance imagery. PLoS One. 2010; doi:10.1371/journal.pone.0012152.
Chen Z, Sadana A. An analysis of antigen-antibody binding kinetics for biosensor applications utilized as a model system: influence of non-specific binding. Biophys Chem. 1996; 57(2–3):177–87.
Sutaria M, Sadana A. Dual-fractal analysis for antigenantibody binding kinetics for biosensor applications. Biotechnol Progr. 1997; 13(4):464–73.
Vollmer F, Arnold S, Keng D. Single virus detection from the reactive shift of a whispering-gallery mode. P Natl Acad Sci USA. 2008; 105(52):20701–4.
Polson RC, Vardeny ZV. Spatially mapping random lasing cavities. Opt Lett. 2010; 35(16):2801–3.
Cao H, Ling Y, Xu JY, Burin AL. Probing localized states with spectrally resolved speckle techniques. Phys Rev E. 2002; doi:10.1103/PhysRevE.66.025601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.H., Kim, Y.L. The potential of naturally occurring lasing for biological and chemical sensors. Biomed. Eng. Lett. 4, 201–212 (2014). https://doi.org/10.1007/s13534-014-0155-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-014-0155-x