Abstract
Objective
The present study was carried out to investigate the possible protective role of copper (II) albumin against zinc oxide nanoparticles (ZnONPs) provoked DNA damage and hepatotoxicity in rats.
Methods
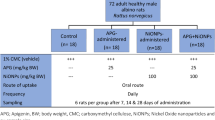
Forty adult male Sprague Dawley rats were randomly divided into five groups; Group I: the control group maintained on a regular diet, Group II: received 1 ml/day of milk as the solvent of Cu (II) albumin complex, Group III: received Cu (II) albumin at 0.03 µg/gm, Group IV: exposed to ZnONPs (400 mg/kg/day), and Group V: exposed to 400 mg/kg/day ZnONPs plus Cu (II) albumin. All treatments were administered for 30 days. At the end of the experiment, animals were euthanized for collection of blood and liver samples. DNA damage in blood and liver was evaluated by using comet assay, while hepatotoxicity was evaluated from histopathological changes of hepatic tissue and liver enzymes (ALT and AST). The oxidative status parameters including nitric oxide (NO), glutathione peroxidase, malondialdehyde (MDA), and total antioxidant capacity (TAC) were also measured.
Results
The results showed that ZnONPs induced oxidative stress through a significant increase in MDA and NO activities, a significant decrease in TAC, and slight decrease in glutathione peroxidase. Significant DNA damage, a significant increase in AST, and a slight increase in ALT were accompanied by histological changes in the liver ZnONPs exposed group. Concurrent Cu (II) albumin supplement to ZnONPs-treated rats in Group IV reversed most of the histopathological changes and DNA damage, significantly lowered ALT and AST levels as well as MDA and NO, and elevated the TAC and GPx.
Conclusion
Based on these results, it can be concluded that Cu (II) albumin effectively protects against ZnONPs-induced hepatic dysfunction and DNA damage in rats.








Similar content being viewed by others
Data availability
Data are available if requested by the journal.
References
Abdel-Magiud DS, Nassar AY, Shatat AR, Mohamed AS (2019) Radical scavenging activities of albumin-copper complex against bromobenzene induced hepatotoxicity. J Clin Toxicol 9(1):1–10
Abo-Hiemad HM, Nassar AY, Shatat AR, Mohamed MA, Soliman M, Abdelrady YA, Sayed AM (2022) Protective effect of copper II-albumin complex against aflatoxin B1- induced hepatocellular toxicity: the impact of Nrf2, PPAR-γ, and NF-kB in these protective effects. J Food Biochem 46(11):e14160. https://doi.org/10.1111/jfbc.14160
Aboulhoda BE, Abdeltawab DA, Rashed LA, Abd Alla MF, Yassa HD (2020) Hepatotoxic effect of oral zinc oxide nanoparticles and the ameliorating role of selenium in rats: a histological, immunohistochemical and molecular study. Tissue Cell 67:101441
Alaraby M, Annangi B, Hernández A, Creus A, Marcos R (2015) A comprehensive study of the harmful effects of ZnO nanoparticles using Drosophila melanogaster as an in vivo model. J Hazard Mater 296:166–174
Almansour MI, Alferah MA, Shraideh ZA, Jarrar BM (2017) Zinc oxide nanoparticles hepatotoxicity: histological and histochemical study. Environ Toxicol Pharmacol 51:124–130
Attia H, Nounou H, Shalaby M (2018) Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxins 6(2), Article 2
Baquial JGL, Sorenson JRJ (1995) Down-regulation of NADPH-diaphorase (nitric oxide synthase) may account for the pharmacological activities of Cu(II)2(3,5-diisopropylsalicylate)4. J Inorg Biochem 60:133–148
Ben-Slama I, Mrad I, Rihane N, Mir LE, Sakly M et al (2015) Sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J Nanomed Nanotechnol 6:284
Berger MM (2006) Antioxidant micronutrients in major trauma and burns: evidence and practice. Nutr Clin Pract 21(5):438–449
Bogiswariy S, Jegathambigai R, Marimuthu K (2008) Effect of acute exposure of cadmium chloride in the morphology of the liver and kidney of mice. In: Proceedings of the international conference on environmental research and technology (ICERT), pp 28–30
Buchtík R, Trávníček Z, Vančo J (2012) In vitro cytotoxicity, DNA cleavage, and SOD-mimic activity of copper(II) mixed-ligand quinolinonato complexes. J Inorg Biochem 116:163–171
Bukhari B, Memon S, Tahir MM, Bhanger MI (2009) Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim Acta Part A Mol Biomol Spectrosc 71(5):1901–1906
Carmona ER, Inostroza-Blancheteau C, Rubio L, Marcos R (2016) Genotoxic and oxidative stress potential of nanosized and bulk zinc oxide particles in Drosophila melanogaster. Toxicol Ind Health 32(12):1987–2001
Chupani L, Niksirat H, Lünsmann V, Haange S-B, von Bergen M, Jehmlich N, Zuskova E (2018) Insight into the modulation of the intestinal proteome of juvenile common carp (Cyprinus carpio L.) after dietary exposure to ZnO nanoparticles. Sci Total Environ 613–614:62–71
Collins AR, Dobson VL, Dušinská M, Kennedy G, Štětina R (1997) The comet assay: what can it really tell us? Mutat Res Fundam Mol Mech Mutagen 375(2):183–193
Dumitru I, Ene CD, Ofiteru AM, Paraschivescu C, Madalan AM, Baciu I, Farcasanu IC (2012) Identification of [CuCl(acac)(tmed)], a copper(II) complex with mixed ligands, as a modulator of Cu, Zn superoxide dismutase (Sod1p) activity in yeast. J Biol Inorg Chem 17(6):961–974
Duncan C, White AR (2012) Copper complexes as therapeutic agents. Metallomics 4(2):127–138. https://doi.org/10.1039/c2mt00174h
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis, and autophagy. Curr Opin Cell Biol 16(6):663–669
El-Saadani MAM (2004) A combination therapy with copper nicotinate complex reduces the adverse effects of 5-fluorouracil on patients with hepatocellular carcinoma. J Exp Therapeut Oncol 4:19–24
El-wafaey DI, Nafea OE, Faruk EM (2022) Naringenin alleviates hepatic injury in zinc oxide nanoparticles exposed rats: impact on oxido-inflammatory stress and apoptotic cell death. Toxicol Mech Methods 32(1):58–66
Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H (2013) Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol 35(1):67–71
Fathi M, Haydari M, Tanha T (2016) Effects of zinc oxide nanoparticles on antioxidant status, serum enzymes activities, biochemical parameters and performance in broiler chickens. J Livestock Sci Technol 4(2):7–13
Friedman RB, Young DS (1989) Effects of disease on clinicallaboratory tests. 4th ed. AACC Press, Washington, D.C.
Ghareeb OA (2021) Toxicopathological effects of zinc oxide nanoparticles on the liver function and preventive role of silymarin in vivo. Indian J Forensic Med Toxicol 15(2):3212–3217
Giordo R, Nasrallah GK, Al-Jamal O, Paliogiannis P, Pintus G (2020) Resveratrol inhibits oxidative stress and prevents mitochondrial damage induced by zinc oxide nanoparticles in zebrafish (Danio rerio). Int J Mol Sci 21(11), Article 11
Gökakın AK, Atabey M, Deveci K, Sancakdar E, Tuzcu M, Duger C, Topcu O (2014) The effects of sildenafil in liver and kidney injury in a rat model of severe scald burn: a biochemical and histopathological study. Ulus Travma Acil Cerrahi Derg 20(5):319–327
Guo Z, Luo Y, Zhang P, Chetwynd AJ, Qunhui Xie H, AbdolahpurMonikh F, Tao W, Xie C, Liu Y, Xu L, Zhang Z, Valsami-Jones E, Lynch I, Zhao B (2020) Deciphering the particle specific effects on metabolism in rat liver and plasma from ZnO nanoparticles versus ionic Zn exposure. Environ Int 136:105437
Hassan ME, Hassan RR, Diab KA, El-Nekeety AA, Hassan NS, Abdel-Wahhab MA (2021) Nanoencapsulation of thyme essential oil: a new avenue to enhance its protective role against oxidative stress and cytotoxicity of zinc oxide nanoparticles in rats. Environ Sci Pollut Res 28(37):52046–52063
Hegazy AA, Ahmed MM, Shehata M (2018) Changes in rats’ liver structure induced by zinc oxide nanoparticles and the possible protective role of vitamin E. Int J Hum Anat 1(3):1–16
Hjiri M, Aida MS, Neri G (2019) NO2 selective sensor based on αFe2O3 nanoparticles synthesized via hydrothermal technique. Sensors 19(1), Article 1
Hosseini SM, Amani R, Moshrefi AH, Razavimehr SV, Aghajanikhah MH, Sokouti Z (2020) Chronic zinc oxide nanoparticles exposure produces hepatic and pancreatic impairment in female rats. Iranian J Toxicol 14(3):145–154
Huang C-C, Aronstam RS, Chen D-R, Huang Y-W (2010) Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol In Vitro 24(1):45–55. https://doi.org/10.1016/j.tiv.2009.09.007
Hussein MMA, Ali HA, Saadeldin IM, Ahmed MM (2016) Querectin alleviates zinc oxide nanoreprotoxicity in male albino rats. J Biochem Mol Toxicol 30(10):489–496
Iakovidis I, Delimaris I, Piperakis SM (2011) Copper and its complexes in medicine: a biochemical approach. Mol Biol Int 2011:1–13
Jeon Y-M, Kim W-J, Lee M-Y (2013) Studies on liver damage induced by nanosized-titanium dioxide in mouse. J Environ Biol 34(2):283–287
Johar D, Roth JC, Bay GH, Walker JN, Kroczak TJ, Los M (2004) Inflammatory response, reactive oxygen species, programmed (necrotic-like and apoptotic) cell death and cancer. Rocz Akad Med Bialymst 1995(49):31–39
Joseph J, Nagashri K, Rani GAB (2013) Synthesis, characterization and antimicrobial activities of copper complexes derived from 4aminoantipyrine derivatives. J Saudi Chem Soc 17(3):285–294
Kang DH (2002) Oxidative stress, DNA damage, and breast cancer. AACN Adv Crit Care 13(4):540–549
Kei S (1978) Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 90(1):37–43
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Kim I, Viswanathan K, Kasi G, Thanakkasaranee S, Sadeghi K, Seo J (2020) ZnO nanostructures in active antibacterial food packaging: preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev Int 0(0):1–29
Klotz L-O, Kröncke K-D, Buchczyk DP, Sies H (2003) Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr 133(5):1448S-1451S
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54(5):356–361
Kumar J, Mitra MD, Hussain A, Kaul G (2019) Exploration of immunomodulatory and protective effect of Withania somnifera on trace metal oxide (zinc oxide nanoparticles) induced toxicity in Balb/c mice. Mol Biol Rep 46(2):2447–2459
Kwon JY, Lee SY, Koedrith P, Lee JY, Kim K-M, Oh J-M, Yang SI, Kim M-K, Lee JK, Jeong J, Maeng EH, Lee BJ, Seo YR (2014) Lack of genotoxic potential of ZnO nanoparticles in in vitro and in vivo tests. Mutat Res Genet Toxicol Environ Mutagen 761:1–9
Landsiedel R, Ma-Hock L, Van Ravenzwaay B, Schulz M, Wiench K, Champ S, Schulte S, Wohlleben W, Oesch F (2010) Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 4(4):364–381
Liao C, Jin Y, Li Y, Tjong SC (2020) Interactions of zinc oxide nanostructures with mammalian cells: cytotoxicity and photocatalytic toxicity. Int J Mol Sci 21(17), Article 17
Mansouri E, Khorsandi L, Orazizadeh M, Jozi Z (2015) Dose-dependent hepatotoxicity effects of zinc oxide nanoparticles. Nanomed J 2(4):273–282
Mir AH, Qamar A, Qadir I, Naqvi AH, Begum R (2020) Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci Rep 10(1):1–14
Moatamed ER, Hussein AA, El-desoky MM, Khayat ZE (2019) Comparative study of zinc oxide nanoparticles and its bulk form on liver function of Wistar rat. Toxicol Ind Health 35(10):627–637
Mohamed AO, Nassar AY, Mandour MAM, Saad Eldien HM, Mohammed GME (2012) Effects of Copper (II) albumin complex on thermal skin burn. Bull Egypt Soc Physiol Sci 32(2):12
Montgomery HACDJ, Dymock JF (1961) Determination of nitrite in water. Analyst 86(102):414
Mukherjee S, Sarathi Mukherjee P (2013) Cu II-azide polynuclear complexes of Cu4 building clusters with Schiff-base co-ligands: synthesis, structures, magnetic behavior and DFT studies. Dalton Trans 42(11):4019–4030
Neyrinck A (2004) Modulation of Kupffer cell activity: physio-pathological consequences on hepatic metabolism. Bull Mem Acad R Med Belg 159(5–6):358–366
Oligny LL, Lough J (1992) Hepatic sinusoidal ectasia. Hum Pathol 23(8):953–956
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Palanimuthu D, Shinde SV, Somasundaram K, Samuelson AG (2013) In vitro and in vivo anticancer activity of copper bis(thiosemicarbazone) complexes. J Med Chem 56(3):722–734
Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya BM (2012) Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health 28(8):675–686
Philips N, Hwang H, Chauhan S, Leonardi D, Gonzalez S (2010) Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res 51(3):224–229
Ramaiah SK (2007) A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45:1551–1557
Sadauskas E, Wallin H, Stoltenberg M, Vogel U, Doering P, Larsen A, Danscher G (2007) Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol 4(1):10
Salama RHM, Nassar AYA, Nafady AAM, Mohamed HHT (2007) A novel therapeutic drug (copper nicotinic acid complex) for non-alcoholic fatty liver. Liver Int 27:454–464
Sasaki YF, Izumiyama F, Nishidate E, Matsusaka N, Tsuda S (1997) Detection of rodent liver carcinogen genotoxicity by the alkaline single-cell gel electrophoresis (Comet) assay in multiple mouse organs (liver, lung, spleen, kidney, and bone marrow)1This paper is dedicated to the memory of the late Prof. Kiyosi Tutikawa, National Institute of Genetics, Mishima, Japan.1. Mutat Res Genet Toxicol Environ Mutagen 391(3):201–214
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF (2010) Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed Nanobiotechnol 2(5):544–568
Sharma V, Singh P, Pandey AK, Dhawan A (2012) Induction of oxidative stress, DNA damage and apoptosis in mouse liver after subacute oral exposure to zinc oxide nanoparticles. Mut Res Genet Toxicol Environ Mutagen 745(1):84–91
Shatat AR, Eldien HMS, Nassar MY, Mohamed AO, Hussein AHM, El-Adasy A-BA, Khames AA, Nassar AY (2013) Protective effects of copper (I)-nicotinate complex against aflatoxicosis. Open Toxicol J 6(1):1–10
Singh SP, Lars B, Luca V, Jian C, Falck JR, Attallah K et al (2016) Downregulation of PGC-1α prevents the beneficial effect of EET-heme oxygenase-1 on mitochondrial integrity and associated metabolic function in obese mice. J Nutr Metab 2016:1–15. https://doi.org/10.1155/2016/9039754
Spaggiari D, Geiser L, Daali Y, Rudaz S (2014) A cocktail approach for assessing the in vitro activity of human cytochrome P450s: an overview of current methodologies. J Pharm Biomed Anal 101:221–237
Surekha P, Kishore AS, Srinivas A, Selvam G, Goparaju A, Reddy PN, Murthy PB (2012) Repeated dose dermal toxicity study of nano zinc oxide with Sprague-Dawley rats. Cutan Ocul Toxicol 31(1):26–32
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques E-Book. 7th ed. Elsevier Health Sciences, p 75–100
Syama S, Sreekanth PJ, Varma HK, Mohanan PV (2014) Zinc oxide nanoparticles induced oxidative stress in mouse bone marrow mesenchymal stem cells. Toxicol Mech Methods 24(9):644–653
Tang H-Q, Xu M, Rong Q, Jin R-W, Liu Q-J, Li Y-L (2016) The effect of ZnO nanoparticles on liver function in rats. Int J Nanomed 11:4275–4285
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35(3):206–221
Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Brenner DE (2009) Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 8(1):113–117
Valentini X, Rugira P, Frau A, Tagliatti V, Conotte R, Laurent S et al (2019) Hepatic and renal toxicity induced by TiO2 nanoparticles in rats: a morphological and metabonomic study. J Toxicol 2019:5767012. https://doi.org/10.1155/2019/5767012
Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA (2002) High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51(6):1889–1895
Wang B, Feng W, Wang M et al (2008) Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res 10:263–276. https://doi.org/10.1007/s11051-007-9245-3
Wang L, Xu H, Qiu Y, Liu X, Huang W, Yan N, Qu Z (2020) Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water. J Hazard Mater 389:121824
Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM (2002) Copper complexes of nonsteroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev 232(1):95–126
Yahya RAM, Azab AE, Shkal KEM, Jm J (2019) Hepatotoxicity induced by copper oxide and zinc oxide nanoparticles and their mixtures in male albino rats. East Afr Sch J Med Sci 2:11
Yao Y, Zang Y, Qu J, Tang M, Zhang T (2019) The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int J Nanomed 14:8787–8804
Yakubu OE, Olawale O, Arowora KA et al (2017) Biochemical changes in haematological and liver function parameters in intoxicated male albino rats treated with hymenocardia acida leaves ethanolic extract. Insights Biomed 2(2):10
Yousef MI, Mutar TF, Kamel MAE-N (2019) Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep 6:336–346
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AMA was involved in preparation of the protocol, performed the experiment, collection of samples, statistical analysis, and writing the article. HF contributed to preparation of the protocol, writing, and revision of the manuscript. DY was involved in comet assay analysis, writing and revision of the manuscript, data analysis, and interpretation of results. MFA contributed to sample collection and preparation for histological examination, photographing, and interpretation of results. AYN was involved in preparation of Copper (II) Albumin Complex. DA contributed to data analysis, preparation, and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Aya M. Abdelnaem, Hala Fathy, Doha Yahia, Marwa F. Ali, Ahmed Y. Nassar, Doaa Almaz declare that they have no conflict of interest.
Consent to participate
All authors participated in the preparation of the current study.
Consent to publish
All authors agree to publish this article.
Ethical approval
The study protocol was approved by the ethical committee of the Faculty of Medicine, Assiut University, Egypt (No. 17101068,4-2020).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelnaem, A.M., Fathy, H., Yahia, D. et al. Ameliorative effects of Copper(II) albumin complex against zinc oxide nanoparticles induced oxidative DNA damage in Sprague Dawley rats. Toxicol. Environ. Health Sci. (2024). https://doi.org/10.1007/s13530-024-00208-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13530-024-00208-w