Abstract
Objective
Testicular tissues and sperms are typically vulnerable to oxidative stress and inflammation. Despite functioning as a signaling molecule in different physiological and pathological processes, hydrogen sulfide (H2S) role in the reproductive system is not fully recognized. This study aimed to assess whether H2S could counteract the testicular damage that acrylamide (AC) causes in male rats.
Methods
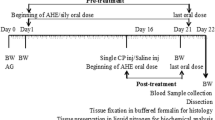
Forty male rats were equally divided randomly into four groups: normal control, AC, H2S, and AC + H2S who received normal saline, 40 mg/Kg of AC, 200 µg/Kg of sodium hydrosulfide NaHS (H2S donor), and 40 mg/Kg of AC + 200 µg/Kg of NaHS by intraperitoneal injection for 14 consecutive days, respectively. Body and testes weights, sperm count and motility, lactate dehydrogenase isoenzyme-x (LDH-X), serum testosterone level, oxidative parameters, the expression level of inducible nitric oxide synthase (iNOS), inflammatory cytokines, and histopathological alterations were evaluated.
Results
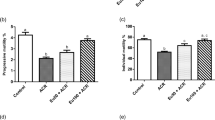
The reduction in relative testicular weights, sperm count, and motility served as evidence of the harmful effects of AC. However, these values were reversed when H2S and AC were combined. Additionally, AC significantly decreased serum testosterone level, testicular LDH-X activity, superoxide dismutase, catalase, and reduced glutathione. While malondialdehyde, expression levels of iNOS protein and inflammatory cytokines (TNF-α, IL-1β, and IL-6) levels were elevated. Interestingly, the co-administration of H2S with AC reversed these values, demonstrating an opposing effect in the previous parameters.
Conclusion
H2S exhibited a protective effect in the rat model of testicular toxicity induced by AC, which may be associated with the suppression of iNOS expression, proinflammatory cytokines, and the inhibition of oxidative stress injury.






Similar content being viewed by others
Data availability
Data available on request from corresponding author.The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
References
Braun O, Coquery C, Kieffer J, Blondel F, Favero C, Besset C, Mesnager J, Voelker F, Delorme C, Matioszek D (2021) Spotlight on the life cycle of acrylamide-based polymers supporting reductions in environmental footprint: review and recent advances. Molecules 27:42. https://doi.org/10.3390/molecules27010042
Lee B-M, Kwon S, Cho YM, Kim K-B, Seo K, Min CS, Kim K (2019) Perspectives on trace chemical safety and chemophobia: risk communication and risk management. J Toxicol Environ Health A 82:186–199. https://doi.org/10.1080/15287394.2019.1618680
Paul V, Ezekiel R, Pandey R (2016) Acrylamide in processed potato products: progress made and present status. Acta Physiol Plant 38:1–23. https://doi.org/10.1007/s11738-016-2290-8
Ciesarová Z, Kukurová K, Torbica A, Belović M, Horváthová J, Daško Ľ, Jelemenská V (2021) Acrylamide and 5-hydroxymethylfurfural in thermally treated non-wheat flours and respective breads. Food Chem 365:130491. https://doi.org/10.1016/j.foodchem.2021.130491
Kunnel SG, Subramanya S, Satapathy P, Sahoo I, Zameer F (2019) Acrylamide induced toxicity and the propensity of phytochemicals in amelioration: a review. Cent Nervous Syst Agents Med Chem Former Curr Med Chem-Cent Nervous Syst Agents 19:100–113. https://doi.org/10.2174/1871524919666190207160236
Pennisi M, Malaguarnera G, Puglisi V, Vinciguerra L, Vacante M, Malaguarnera M (2013) Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10:3843–3854. https://doi.org/10.3390/ijerph10093843
Kucukler S, Caglayan C, Darendelioğlu E, Kandemir FMJLS (2020) Morin attenuates acrylamide-induced testicular toxicity in rats by regulating the NF-κB, Bax/Bcl-2 and PI3K/Akt/mTOR signaling pathways. Life Sci 261:118301. https://doi.org/10.1016/j.lfs.2020.118301
Kandemir FM, Yıldırım S, Kucukler S, Caglayan C, Darendelioğlu E, Dortbudak MB (2020) Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: a multi-biomarker approach. Food Chem Toxicol 138:111190. https://doi.org/10.1016/j.fct.2020.111190
Al-Qahtani F, Arafah M, Sharma B, Siddiqi N (2017) Effects of alpha lipoic acid on acrylamide-induced hepatotoxicity in rats. Cell Mol Biol 63:1–6. https://doi.org/10.14715/cmb/2017.63.6.1
Pan X, Wu X, Yan D, Peng C, Rao C, Yan H (2018) Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol Lett 288:55–64. https://doi.org/10.1016/j.toxlet.2018.02.002
Dasari S, Ganjayi M, Gonuguntla S, Kothapalli S, Konda P, Basha S, Kutagolla P, Meriga B (2018) Evaluation of biomarkers distress in acryla-mide-induced hepatic and nephrotoxicity of albino wistar rat. Adv Anim Vet Sci 6:427–435. https://doi.org/10.17582/journal.aavs/2018/6.10.427.435
Giuffrè A, Vicente JBJOM, Longevity C (2018) Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid Med Cell Longev. https://doi.org/10.1155/2018/6290931
Calabrese V, Scuto M, Salinaro AT, Dionisio G, Modafferi S, Ontario ML, Greco V, Sciuto S, Schmitt CP, Calabrese EJ, Peters V (2020) Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis. Antioxidants (Basel) 9:1303. https://doi.org/10.3390/antiox9121303
Cao X, Xiong S, Zhou Y, Wu Z, Ding L, Zhu Y, Wood ME, Whiteman M, Moore PK, Bian J-s (2018) Renal protective effect of hydrogen sulfide in cisplatin-induced nephrotoxicity. Antioxid Redox Signal 29:455–470. https://doi.org/10.1089/ars.2017.7157
Fan H-N, Wang H-J, Yang-Dan C-R, Ren L, Wang C, Li Y-F, Deng Y (2013) Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep 7:247–253. https://doi.org/10.3892/mmr.2012.1153
Li Z, Polhemus DJ, Lefer DJ (2018) Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res 123:590–600. https://doi.org/10.1161/CIRCRESAHA.118.311134
Goren I, Köhler Y, Aglan A, Pfeilschifter J, Beck K-F, Frank S (2019) Increase of cystathionine-γ-lyase (CSE) during late wound repair: hydrogen sulfide triggers cytokeratin 10 expression in keratinocytes. Nitric Oxide 87:31–42. https://doi.org/10.1016/j.niox.2019.03.004
Chu P, Lin L, Chen P, Su T, Lin C (2017) Negative association between acrylamide exposure and body composition in adults: NHANES, 2003–2004. Nutr Diabet 7:e246–e246. https://doi.org/10.1038/nutd.2016.48
Burek J, Albee R, Beyer J, Bell T, Carreon R, Morden D, Wade C, Hermann E, Gorzinski S (1980) Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J Environ Pathol Toxicol 4:157–182
Slade E, Williams L, Gagnon JJPR (2018) Hydrogen sulfide suppresses ghrelin secretion in vitro and delays postprandial ghrelin secretion while reducing appetite in mice. Physiol Rep 6:e13870. https://doi.org/10.14814/phy2.13870
Ning JZ, Li W, Cheng F, Rao T, Yu WM, Ruan Y, Yuan R, Zhang XB, Du Y, Xiao CC (2018) The protective effects of GYY4137 on ipsilateral testicular injury in experimentally varicocele-induced rats. Exp Ther Med 15:433–439. https://doi.org/10.3892/etm.2017.5417
Yilmaz BO, Yildizbayrak N, Aydin Y, Erkan M (2017) Evidence of acrylamide-and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum Exp Toxicol 36:1225–1235. https://doi.org/10.1177/0960327116686818
Sharpe R, Maddocks S, Millar M, Kerr J, Saunders P, McKinnell C (1992) Testosterone and spermatogenesis identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl 13:172–184
Wang J, Wang W, Li S, Han Y, Zhang P, Meng G, Xiao Y, Xie L, Wang X, Sha J (2018) Hydrogen sulfide as a potential target in preventing spermatogenic failure and testicular dysfunction. Antioxid Redox Signal 28:1447–1462. https://doi.org/10.1089/ars.2016.6968
Kostic T, Andric S, Maric D, Stojilkovic S, Kovacevic RJJE (1999) Involvement of inducible nitric oxide synthase in stress-impaired testicular steroidogenesis. J Endocrinol 163:409–416. https://doi.org/10.1677/joe.0.1630409
Yildizbayrak N, Erkan M (2018) Acrylamide disrupts the steroidogenic pathway in Leydig cells: possible mechanism of action. Toxicol Environ Chem 100:235–246. https://doi.org/10.1080/02772248.2018.1458231
Chen H-J, Ngowi EE, Qian L, Li T, Qin Y-Z, Zhou J-J, Li K, Ji X-Y, Wu D-D (2021) Role of hydrogen sulfide in the endocrine system. Front Endocrinol. https://doi.org/10.3389/fendo.2021.704620
Khanna S, Lakhera PC, Khandelwal S (2011) Interplay of early biochemical manifestations by cadmium insult in sertoli-germ coculture: an in vitro study. Toxicology 287:46–53. https://doi.org/10.1016/j.tox.2011.05.013
Abd-Ellah M, Aly H, Mokhlis H, Abdel-Aziz A (2016) Quercetin attenuates di-(2-ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum Exp Toxicol 35:232–243. https://doi.org/10.1177/0960327115580602
Oyagbemi AA, Adedara IA, Saba AB, Farombi EO (2010) Role of oxidative stress in reproductive toxicity induced by co-administration of chloramphenicol and multivitamin-haematinics complex in rats. Basic Clin Pharmacol Toxicol 107:703–708. https://doi.org/10.1111/j.1742-7843.2010.00561.x
Corsello T, Komaravelli N, Casola A (2018) Role of hydrogen sulfide in NRF2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 7:129. https://doi.org/10.3390/antiox7100129
Beck K-F, Euler J, Eisel F, Beck M, Köhler Y, Sha LK, von Knethen A, Longen S, Pfeilschifter JJBP (2015) Cytokines induce protein kinase A-mediated signalling by a redox-dependent mechanism in rat renal mesangial cells. Biochem Pharmacol 93:362–369. https://doi.org/10.1016/j.bcp.2014.11.009
O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP (2000) Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 63:1285–1293. https://doi.org/10.1095/biolreprod63.5.1285
Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK (2004) The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90:765–768. https://doi.org/10.1111/j.1471-4159.2004.02617.x
Yang R, Jia Q, Ma S, Cui S, Liu X, Wang Y, Gao Q (2017) Effect of hydrogen sulfide on inducible nitric oxide synthase in kidneys of Type 1 diabetic rats. J Cent South Univ Med Sci 42:389–394. https://doi.org/10.11817/j.issn.1672-7347.2017.04.004
Sanocka D, Jędrzejczak P, Szumała-Kaękol A, Frączek M, Kurpisz M (2003) Male genital tract inflammation: the role of selected interleukins in regulation of pro-oxidant and antioxidant enzymatic substances in seminal plasma. J Androl 24:448–455. https://doi.org/10.1002/j.1939-4640.2003.tb02693.x
Hong CY, Park JH, Ahn RS, Im SY, Choi H-S, Soh J, Mellon SH, Lee KJM, Biology C (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 24:2593–2604. https://doi.org/10.1128/MCB.24.7.2593-2604.2004
Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP (1999) Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. J Interferon Cytokine Res 19:1271–1276. https://doi.org/10.1089/107999099312948
Wallace JL (2007) Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci 28:501–505. https://doi.org/10.1016/j.tips.2007.09.003
Rios E, Szczesny B, Soriano FG, Olah G, Szabo CJ (2015) Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int J Mol Med 35:1741–1746. https://doi.org/10.3892/ijmm.2015.2176
Yassa HA, George SM, Refaiy AERM, Moneim EM (2014) Camellia sinensis (green tea) extract attenuate acrylamide induced testicular damage in albino rats. Environ Toxicol 29:1155–1161. https://doi.org/10.1002/tox.21846
Azarbarz N, Shafiei Seifabadi Z, Moaiedi MZ, Mansouri E (2020) Assessment of the effect of sodium hydrogen sulfide (hydrogen sulfide donor) on cisplatin-induced testicular toxicity in rats. Environ Sci Pollut Res 27:8119–8128. https://doi.org/10.1007/s11356-019-07266-5
Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, Sener G, Omurtag GZ (2012) Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep 39:4589–4596. https://doi.org/10.1007/s11033-011-1249-5
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93:141–154. https://doi.org/10.1385/BTER:93:1-3:141
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278. https://doi.org/10.1016/0003-2697(78)90342-1
Beutler E, Yeh MK (1963) Erythrocyte glutathione reductase. Blood 21:573–585. https://doi.org/10.1182/blood.V21.5.573.573
Aebi H (1984) Catalase in vitro. In: Oxygen radicals in biological systems, vol 105. Methods in enzymology. Academic Press, pp 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Abdel-Bakky MS, Hammad MA, Walker LA, Ashfaq MK (2011) Silencing of tissue factor by antisense deoxyoligonucleotide prevents monocrotaline/LPS renal injury in mice. Arch Toxicol 85:1245–1256. https://doi.org/10.1007/s00204-011-0663-8
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Sciences. https://doi.org/10.1016/C2015-0-00143-5
Hozyen HF, Khalil HMA, Ghandour RA, Al-Mokaddem AK, Amer MS, Azouz RA (2020) Nano selenium protects against deltamethrin-induced reproductive toxicity in male rats. Toxicol Appl Pharmacol 408:115274. https://doi.org/10.1016/j.taap.2020.115274
Johnsen SG (1970) Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25. https://doi.org/10.1159/000178170
Mirhoseini M, Talebpour Amiri F, Karimpour Malekshah AA, Rezanejad Gatabi Z, Ghaffari E (2017) Protective effects of melatonin on testis histology following acute torsion-detorsion in rats. Int J Reprod Biomed 15:141–146. https://doi.org/10.29252/ijrm.15.3.141
Acknowledgements
The authors acknowledge with thanks Faculty of Pharmacy, Al-Azhar University, for technical and financial support. This study did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
HAM, AA conceived and designed the study. HAM, MHR, IGS, MGE, AAE, AA performed the practical experiments. EGK, MHG collected and analyzed data. HAM, MGE, AAE, AA, HSG wrote and revised the manuscript: All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hamada Ahmed Mokhlis, Mohammed Helmy Rashed, Ibrahim Ghalib Saleh, Mahmoud Gomaa Eldeib, Ahmed A. El-Husseiny, Emad Gamil Khidr, Maher H. Gomaa, Hesham S. Gad, and Ahmed Aglan declare that they have no conflicts of interest.
Ethical approval
All experimental procedures for animals in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources for the National Research Council. Approval by the Institutional Animal Care and Use Committee of the Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt was obtained before the animal experiments were performed (Azhar-Pharmacy-2022–005).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhlis, H.A., Rashed, M.H., Saleh, I.G. et al. Hydrogen sulfide alleviates acrylamide-induced testicular toxicity in male rats. Toxicol. Environ. Health Sci. 15, 41–51 (2023). https://doi.org/10.1007/s13530-022-00156-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-022-00156-3