Abstract
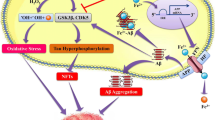
Alzheimer’s disease is an increasing neurodegenerative disease in the aging population. The disease is associated with toxic chemicals of industrial origin. Industrial processes can result in airborne contamination such as fine dust, and water and soil contamination. In the processes, heavy metals are one of the major environmental pollutants. Also, heavy metals are widely used for many appliances. In particular, heavy metals are seriously toxic to the neural system. In several studies, researchers have emphasized the toxicity of heavy metals such as lead, mercury, and cadmium, as a cause of neurofibrillary tangles, aggregation amyloid beta peptides (AβPs) as well as neuronal cell loss. Based on neurotoxic studies showing that heavy metals induce Alzheimer’s disease, this paper discusses molecular mechanisms by which exposure to heavy metals contributes to the pathogenesis of Alzheimer’s disease. Also, we indicate pathway for heavy metal related Alzheimer’s through integrated analysis based on molecular networks. We suggest that the study of signaling networks contributes to our ability to select significant factors for curing heavy metal induced Alzheimer’s disease.
Similar content being viewed by others
References
Srianujata, S. Lead-the toxic metal to stay with human. J. Toxicol. Sci. 23, 237–240 (1998).
Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 56, 174–179 (2003).
Godt, J. et al. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1, doi:10.1186/1745-6673-1-22 (2006).
Goyer, R. A. Lead toxicity: from overt to subclinical to subtle health effects. Environ. Health Perspect. 86, 177–181 (1990).
Flora, G., Gupta, D. & Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 5, 47–58 (2012).
Pelletier, L. et al. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J. Immunol. 140, 750–754 (1988).
Torres, A. M. et al. Deletion of Multispecific Organic Anion Transporter Oat1/Slc22a6 Protects against Mercury-induced Kidney Injury. J. Biol. Chem. 286, 26391–26395 (2011).
Flick, D. F., Kraybill, H. F. & Dimitroff, J. M. Toxic effects of cadmium: A review. Environ. Res. 4, 71–85 (1971).
Huang, J., Tanii, H., Kato, K. & Hashimoto, K. Neuron and glial cell marker proteins as indicators of heavy metal-induced neurotoxicity in neuroblastoma and glioma cell lines. Arch. Toxicol. 67, 491–496 (1993).
Lee, Y. W., Ha, M. S. & Kim, Y. K. Role of Reactive Oxygen Species and Glutathione in Inorganic Mercury-Induced Injury in Human Glioma Cells. Neurochem. Res. 26, 1187–1193 (2001).
Olivieri, G. et al. The effects of β-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and β-amyloid secretion. Neuroscience 113, 849–855 (2002).
Latinwo, L. M. et al. Comparative studies of in vivo genotoxic effects of cadmium chloride in rat brain, kidney and liver cells. Cell Mol. Biol. (Noisy-le-grand) 43, 203–210 (1997).
Xu, F. et al. Mercury-induced toxicity of rat cortical neurons is mediated through N-methyl-D-Aspartate receptors. Mol. Brain. 5, doi: 10.1186/1756-6606-5-30 (2012).
Bokara, K. K. et al. Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. BioMetals 21, 9–16 (2008).
Pieper, I. et al. Mechanisms of Hg species induced toxicity in cultured human astrocytes: genotoxicity and DNA-damage response. Metallomics 6, 662–671 (2014).
Rodríguez, V. M., Jiménez-Capdeville, M. E. & Giordano, M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 145, 1–18 (2003).
Garza, A., Vega, R. & Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 12, RA57-65 (2006).
Pourabdian, S., Eizadi-Mood, N., Golshiri, P. & Amini, F. The Relationship between Blood Lead Level and Neuro-psychological and Hematological Findings in Lead-Exposed Workers of Battery Industry. Iran. J. Toxicol. 5, 521–526 (2011).
Bakulski, K. M. et al. Alzheimer’s Disease and Environmental Exposure to Lead: The Epidemiologic Evidence and Potential Role of Epigenetics. Curr. Alzheimer Res. 9, 563–573 (2012).
Sharma, S. V. et al. Lead (Pb) Toxicity Trigger Schizophrenia in Battery Workers of North Region of India. JNND 2, doi:10.15744/2454-4981.2.302 (2015).
Matte, T. D. et al. Lead Poisoning among Household Members Exposed to Lead-Acid Battery Repair Shops in Kingston, Jamaica. Int. J. Epidemiol. 18, 874–881 (1989).
Hock, C. et al. Increased blood mercury levels in patients with Alzheimer’s disease. J. Neural. Transm (Vienna) 105, 59–68 (1998).
Ehmann, W. D. et al. Brain trace elements in Alzheimer’s disease. Neurotoxicology 7, 195–206 (1986).
Thompson, C. M. et al. Regional brain trace-element studies in Alzheimer’s disease. Neurotoxicology 9, 1–7 (1988).
Mano, Y., Takayanagi, T., Ishitani, A. & Hirota, T. Mercury in hair of patients with ALS. Rinsho. Shinkeigaku 29, 844–848 (1989).
Zahir, F., Rizwi, S. J., Haq, S. K. & Khan, R. H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 20, 351–360 (2005).
Mutter, J. et al. Does Inorganic Mercury Play a Role in Alzheimer’s Disease? A Systematic Review and an Integrated Molecular Mechanism. J. Alzheimers Dis. 22, 357–374 (2010).
Haut, M. W. et al. Neurobehavioral Effects of Acute Exposure to Inorganic Mercury Vapor. Appl. Neuropsychol. 6, 193–200 (1999).
Panayi, A. E. et al. Determination of cadmium and zinc in Alzheimer’s brain tissue using Inductively Coupled Plasma Mass Spectrometry. J. Neurol. Sci. 195, 1–10 (2002).
Lui, E. et al. Metals and the Liver in Alzheimer’s Disease An Investigation of Hepatic Zinc, Copper, Cadmium, and Metallothionein. J. Am. Geriatr. Soc. 38, 633–639 (1990).
Basun, H., Forssell, L. G., Wetterberg, L. & Winblad, B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural. Transm. Park. Dis. Dement. Sect. 3, 231–258 (1991).
Notarachille, G., Arnesano, F., Calò, V. & Meleleo, D. Heavy metals toxicity: effect of cadmium ions on amyloid beta protein 1-42. Possible implications for Alzheimer’s disease. BioMetals 27, 371–388 (2014).
Hart, R. P., Rose, C. S. & Hamer, R. M. Neuropsycological effect of occupational exposure to cadmium. J. Clin. Exp. Neuropsychol. 11, 933–943 (1989).
Li, X. et al. The effect of cadmium on Aβ levels in APP/PS1 transgenic mice. Exp. Ther. Med. 4, 125–130 (2012).
Mao, P. & Reddy, P. H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta. 1812, 1359–1370 (2011).
Chen, C. et al. Increased oxidative DNA damage, as assessed by urinary 8-hydroxy-2'-deoxyguanosine concentrations, and serum redox status in persons exposed to mercury. Clin. Chem. 51, 759–767 (2005).
Hamilton, M. L. et al. A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic. Acids. Res. 29, 2117–2126 (2001).
Sedelnikova, O. A. et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170 (2004).
von Figura, G., Hartmann, D., Song, Z. & Rudolph, K. L. Role of telomere dysfunction in aging and its detection by biomarkers. J. Mol. Med. 87, 1165–1171 (2009).
Bolin, C. M. et al. Exposure to lead (Pb) and the developmental origin of oxidative DNA damage in the aging brain. FASEB J. 20, 788–790 (2006).
Bains, J. S. & Shaw, C. A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain. Res. Brain. Res. Rev. 25, 335–358 (1997).
Selkoe, D. J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu. Rev. Cell. Biol. 10, 373–403 (1994).
Wu, J. et al. Alzheimer’s Disease (AD)-Like Pathology in Aged Monkeys after Infantile Exposure to Environmental Metal Lead (Pb): Evidence for a Developmental Origin and Environmental Link for AD. J. Neurosci. 28, 3–9 (2008).
Basha, M. R. et al. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 25, 823–829 (2005).
Bihaqi, S. W., Huang, H., Wu, J. & Zawia, N. H. Infant Exposure to Lead (Pb) and Epigenetic Modifications in the Aging Primate Brain: Implications for Alzheimer’s Disease. J. Alzheimers. Dis. 27, 819–833 (2011).
Davey, F. D. & Breen, K. C. The interactions between chronic low-level lead and the amyloid β-precursor protein. Amyloid 5, 90–98 (1998).
Mutter, J. et al. Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro. Endocrinol. Lett. 25, 331–339 (2004).
Cedrola, S. et al. Inorganic mercury changes the fate of murine CNS stem cells. FASEB J. 17, 869–871 (2003).
Kosik, K. S., Joachim, C. L. & Selkoe, D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 83, 4044–4048 (1986).
Olivieri, G. et al. Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem. 74, 231–236 (2000).
Busciglio, J., Lorenzo, A., Yeh, J. & Yankner, B. A. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888 (1995).
Mao, P. & Reddy, P. H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim. Biophys. Acta. 1812, 1359–1370 (2011).
Goedert, M. et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–26 (1989).
Zheng, W. H. et al. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115, 201–211 (2002).
Huang, H. C. & Jiang, Z. F. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease. J. Alzheimers Dis. 16, 15–27 (2009).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2010).
Monnet-Tschudi, F. et al. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev. Environ. Health. 21, 105–117 (2006).
Charleston, J. S. et al. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicular is following long-term subclinical methylmercury exposure. Neurotoxicology 17, 127–138 (1996).
Smedman, M. et al. Effects of cadmium, copper, and zinc and beta APP processing and turnover in COS-7 and PC12 cells. Relationship to Alzheimer disease pathology. Mol. Chem. Neuropathol. 31, 13–28 (1997).
Eskes, C., Honegger, P., Juillerat-Jeanneret, L. & MonnetTschudi, F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia 37, 43–52 (2002).
Kawarabyashi, T. et al. Expression of APP in the early stage of brain damage. Brain. Res. 563, 334–338 (1991).
Götz, J., Chen, F., van Dorpe, J. & Nitsch, R. M. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293, 1491–1495 (2001).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Oddo, S. et al. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 24, 1063–1070 (2003).
Syme, C. D. & Viles, J. H. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Abeta) of Alzheimer’s disease. Biochim. Biophys. Acta 1764, 246–256 (2006).
Yano, K. et al. Aggregations of amyloid beta-proteins in the presence of metal ions. Toxicol. Lett. 144, doi: org/10.1016/S0378-4274(03)90499-1 (2003).
Jiang, L. F. et al. Impacts of Cd (II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta 1774, 1414–1421 (2007).
Butterfield, D. A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 36, 1307–1313 (2002).
Drake, J., Link, C. D. & Butterfield, D. A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 24, 415–420 (2003).
Selkoe, D. J. Clearing the brain’s amyloid cobwebs. Neuron 25, 177–180 (2001).
Del Pino, J. et al. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch. Toxicol. 90, 1081–1092 (2016).
Takashima, A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 9, 309–317 (2006).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112, 415–422 (2003).
Nakanishi, A. et al. BRCA1 and p53 tumor suppressor molecules in Alzheimer’s disease. Int. J. Mol. Sci. 16, 2879–2892 (2015).
Liu, H. et al. SB216763, a selective small molecule inhibitor of glycogen synthase kinase-3, improves bleomycin-induced pulmonary fibrosis via activating autophagy. Acta Pharmacol. Sin. 3, 226–233 (2013).
Ling, Y. H., Tornos, C. & Perez-Soler, R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J. Biol. Chem. 273, 18984–18991 (1998).
Ju, T. C., Chen, S. D., Liu, C. C. & Yang, D. I. Protective effects of S-nitrosoglutathione against amyloid β-peptide neurotoxicity. Free. Radic. Biol. Med. 38, 938–949 (2005).
Lin, C. F. et al. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J. Cell. Sci. 120, 2935–2943 (2007).
Lee, H. E. et al. Inhibition of specificity protein 1 by dibenzylideneacetone, a curcumin analogue, induces apoptosis in mucoepidermoid carcinomas and tumor xenografts through Bim and truncated Bid. Oral. Oncol. 50, 189–195 (2013).
Feng, J., Meng, C. & Xing, D. Aβ induces PUMA activation: a new mechanism for Aβ-mediated neuronal apoptosis. Neurobiol. Aging 36, 789–800 (2014).
Li, C. et al. The β isoform of GSK3 mediates podocyte autonomous injury in proteinuric glomerulopathy. J. Pathol. 239, 23–35 (2016).
Stephen, T. L. et al. Effect of B7-2 and CD40 signals from activated antigen-presenting cells on the ability of zwitterionic polysaccharides to induce T-Cell stimulation. Infect. Immun. 73, 2184–2189 (2005).
McQuillan, K., Lynch, M. A. & Mills, K. H. G. Activation of mixed glia by Aβ-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav. Immun. 24, 598–607 (2010).
Wu, K. et al. Cell fate determination factor DACH1 inhibits c-Jun-induced contact-independent growth. Mol. Biol. Cell. 18, 755–767 (2007).
Saini, M. K. & Sanyal, S. N. PTEN regulates apoptotic cell death through PI3-K/Akt/GSK3β signaling pathway in DMH induced early colon carcinogenesis in rat. Exp. Mol. Pathol. 93, 135–146 (2012).
Kam, T.-I. et al. FcγRIIb mediates amyloid-β neurotoxicity and memory impairment in Alzheimer’s disease. J. Clin. Invest. 123, 2791–2802 (2013).
Ekström, L. et al. Basal expression of the human MAPEG members microsomal glutathione transferase 1 and prostaglandin E synthase genes is mediated by Sp1 and Sp3. Biochim. Biophys. Acta 1627, 79–84. (2003).
Satoh, K. et al. Expression of prostaglandin E synthase mRNA is induced in beta-amyloid treated rat astrocytes. Neurosci. Lett. 283, 221–223 (2000).
de Oliveira, A. C. et al. Pharmacological inhibition of Akt and downstream pathways modulates the expression of COX-2 and mPGES-1 in activated microglia. J. Neuroinflammation 9, doi: 10.1186/1742-2094-9-2 (2012).
Fiala, M. et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA. 104, 12849–12854 (2007).
Wasiluk, K. R., McCulloch, K. A., Banton, K. L. & Dunn, D. L. Sp1 elements regulate transcriptional activity within the murine Toll-like receptor 4 promoter. Surg. Infect. (Larchmt) 7, 489–499 (2006).
Bai, X. T., Baydoun, H. H. & Nicot, C. HTLV-I p30: A versatile protein modulating virus replication and pathogenesis. Mol. Aspects Med. 31, 344–349 (2010).
Stengel, C. et al. In vivo and in vitro properties of STX2484: a novel non-steroidal anti-cancer compound active in taxane-resistant breast cancer. Br. J. Cancer. 111, 300–308 (2014).
Jans, D. A., Thomas, R. J. & Gillespie, M. T. Parathyroid Hormone-Related Protein (PTHrP): A Nucleocytoplasmic Shuttling Protein with Distinct Paracrine and Intracrine Roles. Vitam. Horm. 66, 345–384 (2003).
Kaminsky, Y. G., Marlatt, M. W., Smith, M. A. & Kosenko, E. A. Subcellular and metabolic examination of amyloid-β peptides in Alzheimer disease pathogenesis: Evidence for Aβ25-35. Exp. Neurol. 221, 26–37 (2010).
Wang, W., Gu, L., Verkhratsky, A. & Peng, L. Ammonium Increases TRPC1 Expression Via Cav-1/PTEN/AKT/GSK3β Pathway. Neurochem. Res. 42, 762–776 (2016).
Linde, C. I., Baryshnikov, S. G., Mazzocco-Spezzia, A. & Golovina, V. A. Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein. Am. J. Physiol. Cell Physiol. 300, C1502–1512 (2011).
de Souza, L. B. & Ambudkar, I. S. Trafficking mechanisms and regulation of TRPC channels. Cell Calcium 56, 43–50 (2014).
Corsini, N. S. et al. The Death Receptor CD95 Activates Adult Neural Stem Cells for Working Memory Formation and Brain Repair. Cell Stem Cell 5, 178–190 (2009).
Chen, Y. & Dong, C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 16, 386–394 (2009).
Edelman, M. J. & Shvartsbeyn, M. Epothilones in Development for Non-Small-Cell Lung Cancer: Novel Anti-Tubulin Agents with the Potential to Overcome Taxane Resistance. Clin. Lung Cancer 13, 171–180 (2012).
Gupta, C., Kaur, J. & Tikoo, K. Regulation of MDAMB-231 cell proliferation by GSK-3β involves epigenetic modifications under high glucose conditions. Exp. Cell Res. 324, 75–83 (2014).
Ghosh, F., Arnér, K. & Engelsberg, K. Isolation of photoreceptors in the cultured full-thickness fetal rat retina. Invest. Ophthalmol. Vis. Sci. 50, 826–835 (2009).
Levin, E. C. et al. Neuronal expression of vimentin in the Alzheimer’s disease brain may be part of a generalized dendritic damage-response mechanism. Brain Res. 1298, 194–207 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.J., Park, M.K. & Seo, Y.R. Pathogenic Mechanisms of Heavy Metal Induced-Alzheimer’s Disease. Toxicol. Environ. Health Sci. 10, 1–10 (2018). https://doi.org/10.1007/s13530-018-0340-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-018-0340-x