Abstract
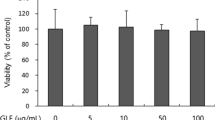
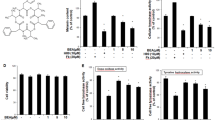
Umbelliferone (7-hydroxycoumarin) treatment caused an increase in melanin content in B16F10 melanoma cells in a dose-dependent manner, without causing toxicity. The increase in melanin content was correlated with increases in the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis, and the expressions of melanogenic proteins, including tyrosinase, tyrosinase-related protein 1, and microphthalmia-associated transcription factor, the master transcriptional regulator for melanogenesis. Unlike α-melanocyte-stimulating hormone, umbelliferone did not cause melanogenesis-associated oxidation and depletion of glutathione. Conversely, umbelliferone treatment resulted in a significant and dose-dependent increase in glutathione. Umbelliferone caused activation of JNK, p38 MAPK, and GSK3β in a dose- dependent manner, suggesting possible involvement of those protein kinases in umbelliferone-induced stimulations of melanogenesis and antioxidant system. Our results suggest that umbelliferone stimulates both melanogenesis and antioxidant defense, providing more effective protection against UV-induced photodamage. They also imply possible applications of umbelliferone in self-tanning and treatment of skin disorders related with melanin deficiency.
Similar content being viewed by others
References
Yamaguchi, Y., Brenner, M. & Hearing, V. J. The regulation of skin pigmentation. J. Biol. Chem. 282, 27557–27561 (2007).
Brenner, M. & Hearing, V. J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 84, 539–549 (2008).
Hearing, V. J. Determination of melanin synthetic pathways. J. Invest. Dermatol. 131, E8–E11 (2011).
Videira, I. F., Moura, D. F. & Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 88, 76–83 (2013).
D’Mello, S. A., Finlay, G. J., Baguley, B. C. & Askarian-Amiri, M. E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 17, 1144; doi:10.3390/ijms17071144 (2016).
Busca, R. & Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment. Cell Res. 13, 60–69 (2000).
Park, H. Y., Kosmadaki, M., Yaar, M. & Gilchrest, B. A. Cellular mechanisms regulating human melanogenesis. Cell Mol. Life Sci. 66, 1493–1506 (2009).
Goding, C. R. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 14, 1712–1728 (2000).
Saha, B. et al. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 19, 595–605 (2006).
Atkinson, J. M. et al. Activating the Wnt/beta-Catenin Pathway for the Treatment of Melanoma-Application of LY2090314, a Novel Selective Inhibitor of Glycogen Synthase Kinase-3. PLoS One 10, e0125028 (2015).
Venugopala, K. N., Rashmi, V. & Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 963248 (2013).
Kabeya, L. M. et al. 7-Hydroxycoumarin modulates the oxidative metabolism, degranulation and microbial killing of human neutrophils. Chem. Biol Interact. 206, 63–75 (2013).
Mercolini, L. et al. Quantitative evaluation of auraptene and umbelliferone, chemopreventive coumarins in citrus fruits, by HPLC-UV-FL-MS. J. Agric. Food Chem. 61, 1694–1701 (2013).
Karthikeyan, R. et al. 7-Hydroxycoumarin prevents UVB-induced activation of NF-kappaB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. J. Photochem. Photobiol. B 161, 170–176 (2016).
Asthana, S. et al. Structure-activity relationship study of hydroxycoumarins and mushroom tyrosinase. J. Agric. Food Chem. 63, 7236–7244 (2015).
Lacour, J. P., Gordon, P. R., Eller, M., Bhawan, J. & Gilchrest, B. A. Cytoskeletal events underlying dendrite formation by cultured pigment cells. J. Cell Physiol. 151, 287–299 (1992).
Romero, F. J., Zukowski, D. & Mueller-Klieser, W. Glutathione content of V79 cells in two-or three-dimensional culture. Am. J. Physiol. 272, C1507–1512 (1997).
Mastore, M., Kohler, L. & Nappi, A. J. Production and utilization of hydrogen peroxide associated with melanogenesis and tyrosinase-mediated oxidations of DOPA and dopamine. FEBS J. 272, 2407–2415 (2005).
Kayatz, P. et al. Oxidation causes melanin fluorescence. Invest. Ophthalmol. Vis. Sci. 42, 241–246 (2001).
Wakamatsu, K., Nakanishi, Y., Miyazaki, N., Kolbe, L. & Ito, S. UVA-induced oxidative degradation of melanins: fission of indole moiety in eumelanin and conversion to benzothiazole moiety in pheomelanin. Pigment Cell Melanoma Res. 25, 434–445 (2012).
Maresca, V. et al. Correlation between melanogenic and catalase activity in in vitro human melanocytes: a synergic strategy against oxidative stress. Pigment Cell Melanoma Res. 21, 200–205 (2008).
Jenkins, N. C. & Grossman, D. Role of melanin in melanocyte dysregulation of reactive oxygen species. Biomed. Res. Int. 2013, 908797 (2013).
Lin, J. Y. & Fisher, D. E. Melanocyte biology and skin pigmentation. Nature 445, 843–850 (2007).
Mouret, S., Forestier, A. & Douki, T. The specificity of UVA-induced DNA damage in human melanocytes. Photochem. Photobiol. Sci. 11, 155–162 (2012).
Swalwell, H., Latimer, J., Haywood, R. M. & Birch-Machin, M. A. Investigating the role of melanin in UVA/UVB-and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic Biol. Med. 52, 626–634 (2012).
Denat, L., Kadekaro, A. L., Marrot, L., Leachman, S. A. & Abdel-Malek, Z. A. Melanocytes as instigators and victims of oxidative stress. J. Invest. Dermatol. 134, 1512–1518 (2014).
Bickers, D. R. & Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 126, 2565–2575 (2006).
Balasubramanian, S., Efimova, T. & Eckert, R. L. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 277, 1828–1836 (2002).
Balogun, E. et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 371, 887–895 (2003).
Alia, M. et al. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 212, 110–118 (2006).
Selimovic, D., Hassan, M., Haikel, Y. & Hengge, U. R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal 20, 311–322 (2008).
Zhao, L. M. et al. P-hydroxycinnamaldehyde induces B16-F1 melanoma cell differentiation via the RhoA-MAPK signaling pathway. Cell Physiol. Biochem. 38, 2247–2260 (2016).
Baek, S. H. & Lee, S. H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 24, 761–766 (2015).
Peng, H. Y., Lin, C. C., Wang, H. Y., Shih, Y. & Chou, S. T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS One 9, e95186 (2014).
Wu, M. et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 14, 301–312 (2000).
Levy, C., Sonnenblick, A. & Razin, E. Role played by microphthalmia transcription factor phosphorylation and its Zip domain in its transcriptional inhibition by PIAS3. Mol. Cell Biol. 23, 9073–9080 (2003).
Xu, W. et al. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res. 255, 135–143 (2000).
Medina, M. & Wandosell, F. Deconstructing GSK-3: The Fine Regulation of Its Activity. Int. J. Alzheimers Dis. 2011, 479249 (2011).
Qian, W. et al. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J. Alzheimers Dis. 19, 1221–1229 (2010).
Takeda, K. et al. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum. Mol. Genet. 9, 125–132 (2000).
Khaled, M. et al. Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 277, 33690–33697 (2002).
Kakade, V. R. et al. A cAMP and CREB-mediated feed-forward mechanism regulates GSK3beta in polycystic kidney disease. J. Mol. Cell Biol. 8, 464–476 (2016).
Grimes, C. A. & Jope, R. S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65, 391–426 (2001).
Bellei, B., Flori, E., Izzo, E., Maresca, V. & Picardo, M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal 20, 1750–1761 (2008).
Hosoi, J., Abe, E., Suda, T. & Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 45, 1474–1478 (1985).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Neuschwander-Tetri, B. A. & Roll, F. J. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal. Biochem. 179, 236–241 (1989).
Kim, S., You, D. H., Han, T. & Choi, E. M. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B 141C, 301–307 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, Y., Ku, B., Kim, D. et al. Umbelliferone stimulated melanogenesis and increased glutathione level in B16F10 cells. Toxicol. Environ. Health Sci. 9, 152–160 (2017). https://doi.org/10.1007/s13530-017-0316-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-017-0316-2