Abstract
Unobserved heterogeneity in mortality risk is pervasive and consequential. Mortality deceleration—the slowing of mortality’s rise with age—has been considered an important window into heterogeneity that otherwise might be impossible to explore. In this article, I argue that deceleration patterns may reveal surprisingly little about the heterogeneity that putatively produces them. I show that even in a very simple model—one that is composed of just two subpopulations with Gompertz mortality—(1) aggregate mortality can decelerate even while a majority of the cohort is frail; (2) multiple decelerations are possible; and (3) mortality selection can produce acceleration as well as deceleration. Simulations show that these patterns are plausible in model cohorts that in the aggregate resemble cohorts in the Human Mortality Database. I argue that these results challenge some conventional heuristics for understanding the relationship between selection and deceleration; undermine certain inferences from deceleration timing to patterns of social inequality; and imply that standard parametric models, assumed to plateau at most once, may sometimes badly misestimate deceleration timing—even by decades.




Similar content being viewed by others
Notes
Rau et al. (2009) compared the alternatives and advocated this measure.
Lynch and Brown (2001) used the term absolute deceleration as I use it but used relative deceleration to refer to the life table aging rate (LAR), discussed later in Footnote 3. They did not discuss what I call relative deceleration, which entered the demographic literature more recently, with Rau et al. (2009).
The major alternative to the derivatives of mortality, in conceptualizing deceleration, is the slope of the natural log of mortality, dubbed the life table aging rate (LAR) by Horiuchi (e.g., 1997; Horiuchi and Coale 1990; Horiuchi and Wilmoth 1997). Its chief disadvantage for this article is that because the LAR is relative to the overall level of mortality, mortality acceleration/deceleration as measured with the LAR is sensitive to the level of the age-invariant component of mortality (Vaupel and Zhang 2010). In contrast, the mortality derivatives are functions only of the derivatives of cohort frailty composition and of subpopulation mortality (as shown in Online Resource 1). Using the derivatives of mortality in its own scale therefore allows us to focus more cleanly on the contribution of mortality selection: that is, of the declining composition of frail members of the cohort.
Similarly, Lynch and Brown (2001) described the general result that high-mortality populations decelerate at younger ages as follows:
The heterogeneity hypothesis of Horiuchi and Wilmoth (1998) suggests that the age at which deceleration begins should increase over time. The rationale for this prediction is that, as a population becomes more homogeneously robust, the frailer members of the population live longer. Hence their mortality patterns are more similar to that of the most robust subpopulation. This implies a later age before mortality rates come to be governed by the more robust subpopulation, and hence an older age at which deceleration begins. (p. 81; emphasis added)
The results in the present article do not directly speak to this proposal because Heathcote et al.’s model assigns heterogeneous slopes to the subpopulations, whereas the model presented here assumes proportional hazards. This article shows that in the proportional hazards setting, generally considered a more restrictive assumption, even cohorts with only two, not three, closed subpopulations can experience two successive decelerations.
Demographic intuitions on this point may be influenced by a result presented in perhaps the most influential paper introducing mortality selection to a wide demographic audience, Vaupel and Yashin’s (1985) “Heterogeneity’s Ruses.” Vaupel and Yashin (1985:177) wrote:
The sudden decline in the observed hazard rate is produced by the rapid extinction of the frailer subcohort. Until the point of decline, the frailer subcohort experiences death rates that are relatively low. Then, due to the exponential increase in the force of mortality, the death rates become sufficiently large that within a few years almost all of the frailer subcohort dies. The observed hazard rate declines to the level of the hazard rate for the more robust subcohort. Since this hazard rate is increasing, the observed hazard rate then starts to increase as well: the observed hazard rate now equals the hazard rate for the more robust subcohort because only members of the more robust subcohort are still alive.
Vaupel and Yashin were describing a cohort whose mortality increases, decreases, then increases, rather than the acceleration and deceleration of such a cohort. But the vivid imagery of decline precipitated by the rapid extinction of the frail, and then rising with the mortality of the robust, may have been naturally extended by analysts from hazard slopes to higher derivatives. As I will show, the analog of Vaupel and Yashin’s slope pattern in the third derivative of the hazard is one form of deceleration and acceleration that can occur, but only one form—and not at all the most common form in the model cohorts to be considered here.
I give the full parameters of this example and justify their reasonableness in Footnote 16, after I describe the class of simulated cohorts from which this example is drawn.
For a rough-and-ready sense of what extreme mortality differentiation looks like, consider sex differences in Russian mortality. Russian cohorts born in 1872–1980 have an age-specific ratio of the male to female annual mortality rate ranging from 0.77 to 4.87, with a mean (weighted by total exposure) of 2.85. The sex ratio is increasing over time; for cohorts born beginning in 1950, the mean weighted ratio is 3.01, and when limited to ages 50–100, as in the simulations, the ratio for those modern cohorts is 3.19 (author’s calculations from HMD data).
To ensure that no periods of deceleration or reacceleration are censored, I calculate the mortality derivatives up to age 150, by which point the frail are extinct in all cohorts. However, parametric (Gompertz and logistic) models, used for specific purposes described later in the article, are estimated on ages 50–100 to ensure comparability between the models for real and simulated cohorts (since real data do not extend to age 150).
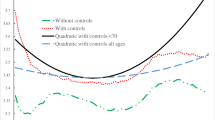
One might be concerned that it is impossible for a cohort to be 75 % frail at age 50 with reasonably valued Gompertz subpopulations because too many frail will have died by age 50. It turns out that this is not the case. Were the subpopulation intercept and slope parameters constant from birth, this would correspond to a proportion frail at birth in the range of .750 to 1 in the final universe of simulated cohorts, with a mean value of .887 (calculations omitted; available upon request). A proportion frail of 1 is incompatible with the assumption of two subpopulations. The 37 cohorts that generate that result, given the assumption of constant lifetime subpopulation mortality parameters, are the cohorts with the lowest
 (slope) and highest
(slope) and highest  (intercept) values in the simulation universe. Excluding them does not appreciably change results.
(intercept) values in the simulation universe. Excluding them does not appreciably change results.Some demographic theory on mortality compression suggests that such populations—in which longevity relative to one’s cohort is the exception rather than the rule—are likely to be disadvantaged populations, insofar as modern health advances have more dramatically altered mortality by raising much of the population to a higher standard length of life than by allowing the most advantaged to live ever longer (e.g., Brown et al. 2012). Thus, it may be among relatively disadvantaged populations that multiple deceleration and high-frailty deceleration may occur. (Insofar as such populations often are the least empirically well documented, it may be especially difficult to amass the data required to circumvent the parametric assumptions shown in this article to sometimes be deeply distorting.)
On the other hand, recent work examining cross-period and cross-cohort mortality variation at a variety of ages shows that although mortality advances reduce variation from birth, such advances may increase variation at older ages, in part because with reduced early-life mortality, more cohort members who are frail live to old age (Engelman et al. 2010). Thus, even if advantaged populations have fewer frail members from birth than disadvantaged populations, they may have as many or more frail members at the elderly ages at which deceleration may occur.
In short, demographic theory does not preclude models with high frailty composition at early-old ages, such as the models explored in this article, for either disadvantaged or advantaged populations.
MATLAB code is available from the author upon request.
Estimating Gompertz models (and, later, logistic models) on the simulated cohorts requires estimating discrete survivorship at each age so that the parametric estimation can be weighted by survivorship, as in real data on individuals. These discrete survivorships are estimated from the mortality functions using standard life table methods that assume constant mortality within each age interval (Preston et al. 2001:46–47). To make this assumption palatable, which violates the assumption of Gompertz subpopulation mortality, I use age increments of only four days.
Human Mortality Database (2011). I use all cohort (vs. period) data included in the HMD.
The constraint that simulated cohorts resemble a real combination of intercept and slope is relevant because of the well-known negative correlation across cohorts between those two parameters, first noted by Strehler and Mildvan (1960) and still of great demographic interest (e.g., Finkelstein 2012; Zheng et al. 2011).
The method used here produces a set of simulated cohorts whose parameters are similar to those of real cohorts because the bulk of the real data fall into a parallelogram-like shape. This is particularly true for simulated cohorts close to relatively recent real cohorts, for which the data are less sparse. All patterns discussed in this article occur across the range of Gompertz intercept and slope values in the HMD universe (also shown visually in Online Resource 2), including among cohorts that fall amid dense clusters of real data.
The example cohort presented in Fig. 1 and Table 2, defined by the parameters f = 5,
 = .002, and β = .103, has Gompertz intercept .009 and slope .081. It was chosen arbitrarily from among those cohorts exhibiting multiple relative decelerations whose aggregate parameters fell in a dense cluster of HMD cohorts, born in Sweden and Denmark in the mid-nineteenth century and in England, Wales, and Scotland in the late nineteenth century.
= .002, and β = .103, has Gompertz intercept .009 and slope .081. It was chosen arbitrarily from among those cohorts exhibiting multiple relative decelerations whose aggregate parameters fell in a dense cluster of HMD cohorts, born in Sweden and Denmark in the mid-nineteenth century and in England, Wales, and Scotland in the late nineteenth century.Deceleration timing for logistic models is defined in the same way as for the nonparametric true hazards—that is, when the second or third derivative become negative—using formulas for those derivatives taken from Rau et al. (2009).
Previous research has found deceleration at very similar ages to those identified (sometimes erroneously) here and changes in deceleration timing that are often much smaller than the potential two-decade error identified here. For example, using logistic models, Rau et al. (2009) found relative deceleration among English and Welsh women at age 93 and absolute deceleration at age 103, and Bebbington et al. (2007) found absolute deceleration among Canadian men at age 92 and women at age 96.5. It stands to reason that similar problems might occur when using arctangent models because of their similarity to logistic models; using arctangent models, Lynch and Brown (2001) found absolute deceleration among U.S. white women and men at ages 95–96 and 93–95, respectively, from 1968 to 1992; and Lynch et al. (2003) found absolute deceleration from 1970 to 1992 for U.S. whites at ages 93–95 and for blacks at ages 92–96 (in unadjusted data) or 101–104 (in data adjusted for potential age misreporting).
Horiuchi and Wilmoth (1998) made a similar contribution for deceleration patterns across causes of death.
Moreover, in practice, many comparative analyses will compare the mortality of cohorts that differ in their frailty distribution at whatever age is taken as baseline. This is because population scientists often wish to compare the mortality of more-advantaged and less-advantaged groups to each other, and cross-national analyses show that even populations with similar life expectancy may differ considerably in their degree of heterogeneity (Edwards and Tuljapurkar 2005). The results in this article suggest that deceleration may occur at several different points in the process of shifting from a relatively frail to an almost entirely robust surviving cohort. When the cohorts also had very different heterogeneity distributions to begin with, inferences from deceleration patterns to the patterns of heterogeneity within each cohort may be particularly problematic.
References
Beard, R. E. (1959). Notes on some mathematical mortality models. In G. E. W. Wolstenholme & M. O’Connor (Eds.), The lifespan of animals (pp. 302–311). Boston, MA: Little, Brown.
Beard, R. E. (1971). Some aspects of theories of mortality, cause of death analysis, forecasting and stochastic processes. In W. Brass (Ed.), Biological aspects of demography (pp. 57–68). London, UK: Taylor & Francis.
Bebbington, M., Lai, C.-D., & Zitikis, R. (2007). Modeling human mortality using mixtures of bathtub shaped failure distributions. Journal of Theoretical Biology, 245, 528–538.
Berkman, L., Singer, B., & Manton, K. (1989). Black/white differences in health status and mortality among the elderly. Demography, 26, 661–678.
Bongaarts, J. (2005). Long-range trends in adult mortality: Models and projection methods. Demography, 42, 23–49.
Brown, D. C., Hayward, M., Montez, J. K., Hidajat, M. M., Hummer, R. A., & Chiu, C.-T. (2012). The significance of education for mortality compression in the United States. Demography, 49, 819–840.
Carey, J. R., Liedo, P., Orozco, D., & Vaupel, J. W. (1992). Slowing of mortality rates at older ages in large medfly cohorts. Science, 258, 457–461.
Costa, D. L. (2012). Scarring and mortality selection among Civil War POWs: A long-term mortality, morbidity, and socioeconomic follow-up. Demography, 49, 1185–1206.
Curtsinger, J. W., Fukui, H. H., Townsend, D. R., & Vaupel, J. W. (1992). Demography of genotypes: Failure of the limited life-span paradigm in Drosophila melanogaster. Science, 258, 461–463.
Drapeau, M. D., Gass, E. K., Simison, M. D., Mueller, L. D., & Rose, M. R. (2000). Testing the heterogeneity theory of late-life mortality plateaus by using cohorts of Drosophila melanogaster. Experimental Gerontology, 35, 71–84.
Dupre, M. E., Franzese, A. T., & Parrado, E. A. (2006). Religious attendance and mortality: Implications for the black-white mortality crossover. Demography, 43, 141–164.
Edwards, R., & Tuljapurkar, S. (2005). Inequality in lifespans and a new perspective on mortality convergence across industrialized countries. Population and Development Review, 31, 645–674.
Engelman, M., Canudas-Romo, V., & Agree, E. (2010). The implications of increased survivorship for mortality variation in aging populations. Population and Development Review, 36, 511–539.
Finkelstein, M. (2012). Discussing the Strehler-Mildvan model of mortality. Demographic Research, 26(article 9), 191–206. doi:10.4054/DemRes.2012.26.9
Fukui, H. H., Xiu, L., & Curtsinger, J. W. (1993). Slowing of age-specific mortality rates in Drosophila melanogaster. Experimental Gerontology, 28, 585–599.
Gampe, J. (2010). Human mortality beyond age 110. In H. Maier, J. Gampe, B. Jeune, J.-M. Robine, & J. Vaupel (Eds.), Demographic Research Monographs 7: Supercentenarians (pp. 219–230). Berlin, Germany: Springer.
Heathcote, C. R., Puza, B. D., & Roberts, S. P. (2009). The use of aggregate data to estimate Gompertz-type old-age mortality in heterogeneous populations. Australian & New Zealand Journal of Statistics, 51, 481–497.
Horiuchi, S. (1997). Postmenopausal acceleration of age-related mortality increase. Journals of Gerontology Series A: Biological Sciences, 52, B78–B92.
Horiuchi, S., & Coale, A. J. (1990). Age patterns of mortality for older women: An analysis using the age-specific rate of mortality change with age. Mathematical Population Studies, 2, 245–267.
Horiuchi, S., & Wilmoth, J. R. (1997). Age patterns of the life table aging rate for major causes of death in Japan, 1951–1990. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 52, B67–B77.
Horiuchi, S., & Wilmoth, J. R. (1998). Deceleration in the age pattern of mortality at older ages. Demography, 35, 391–412.
Human Mortality Database (HMD). (2011). University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Retrieved from http://www.mortality.org or http://www.humanmortality.de
Kannisto, V. (1992). Frailty and survival. Genus, 47, 101–118.
Kestenbaum, B. (1992). A description of the extreme aged population based on improved Medicare enrollment data. Demography, 29, 565–580.
Kulminski, A. M., Ukraintseva, S. V., Akushevich, I. V., Arbeev, K. G., & Yashin, A. I. (2007). Cumulative health deficiencies as a characteristic of long life. Journal of the American Geriatrics Society, 55, 935–940.
Lynch, S. M., & Brown, J. S. (2001). Reconsidering mortality compression and deceleration: An alternative model of mortality rates. Demography, 38, 79–95.
Lynch, S. M., Brown, J. S., & Harmsen, K. G. (2003). Black-white differences in mortality compression and deceleration and the mortality crossover reconsidered. Research on Aging, 25, 456–483.
Manton, K., Poss, S. S., & Wing, S. (1979). The black/white mortality crossover: Investigation from the perspective of the components of aging. The Gerontologist, 19, 291–300.
Masters, R. K. (2012). Uncrossing the U.S. black-white mortality crossover: The role of cohort forces in life course mortality risk. Demography, 49, 773–796.
Missov, T. I., & Finkelstein, M. S. (2011). Admissible mixing distributions for a general class of mixture survival models with known asymptotics. Theoretical Population Biology, 80, 64–70.
Mueller, L. D., Rauser, C. L., & Rose, M. R. (2011). Does aging stop? Oxford, UK: Oxford University Press.
Olshansky, S. J. (1998). On the biodemography of aging: A review essay. Population and Development Review, 24, 381–393.
Palloni, A. (2006). Reproducing inequalities: Luck, wallets, and the enduring effects of childhood health. Demography, 43, 587–615.
Preston, S. H., Heuveline, P., & Guillot, M. (2001). Demography: Measuring and modeling population processes. New York: Wiley-Blackwell.
Rau, R., Muszynska, M., & Baudisch, A. (2009, May). At what age does mortality start to decelerate? Paper presented at the annual meeting of the Population Association of America, Detroit, MI.
Rauser, C. L., Abdel-Aal, Y., Sheih, J. A., Suen, C. W., Mueller, L. D., & Rose, M. R. (2005). Lifelong heterogeneity in fecundity is insufficient to explain late-life fecundity plateaus in Drosophila melanogaster. Experimental Gerontology, 40, 660–670.
Robert, S. A., & House, J. S. (2000). Socioeconomic inequalities in health: An enduring sociological problem. In C. E. Bird, P. Conrad, & A. M. Fremont (Eds.), Handbook of medical sociology (5th ed., pp. 79–97). Upper Saddle River, NJ: Prentice Hall.
Rodriguez, G. (1994). Statistical issues in the analysis of reproductive histories using hazard models. Annals of the New York Academy of Sciences, 709, 266–279.
Rogers, R. G. (2002). Mortality differentials in a diverse society. In N. A. Denton & S. Tolnay (Eds.), American diversity: A demographic challenge for the twenty-first century (pp. 129–154). Albany, NY: SUNY Press.
Steinsaltz, D. (2005). Re-evaluating a test of the heterogeneity explanation for mortality plateaus. Experimental Gerontology, 40, 101–113.
Steinsaltz, D., & Evans, S. N. (2004). Markov mortality models: Implications of quasistationarity and initial distributions. Theoretical Population Biology, 65, 319–337.
Steinsaltz, D. R., & Wachter, K. W. (2006). Understanding mortality rate deceleration and heterogeneity. Mathematical Population Studies, 13, 19–37.
Strehler, B. L., & Mildvan, A. S. (1960). General theory of mortality and aging. Science, 132, 14–21.
Thatcher, A. R., Kannisto, V., & Vaupel, J. W. (1998). The force of mortality at ages 80 to 120. Odense, Denmark: Odense University Press.
Vaupel, J. W. (1997). Trajectories of mortality at advanced ages. In K. W. Wachter & C. E. Finch (Eds.), Between Zeus and the salmon: The biodemography of longevity (pp. 17–37). Washington, DC: National Academy Press.
Vaupel, J. W., & Carey, J. R. (1993). Compositional interpretations of medfly mortality. Science, 260, 1666–1667.
Vaupel, J. W., Manton, K. G., & Stallard, E. (1979). Impact of heterogeneity in individual frailty on the dynamics of mortality. Demography, 16, 439–454.
Vaupel, J. W., & Yashin, A. I. (1985). Heterogeneity’s ruses: Some surprising effects of selection on population dynamics. American Statistician, 39, 176–185.
Vaupel, J. W., & Zhang, Z. (2010). Attrition in heterogeneous cohorts. Demographic Research, 23(article 26), 737–748. doi:10.4054/DemRes.2010.23.26
Wachter, K. W., & Finch, C. E. (Eds.). (1997). Between Zeus and the salmon: The biodemography of longevity. Washington, DC: National Academy Press.
Zajacova, A., Goldman, N., & Rodriguez, G. (2009). Unobserved heterogeneity can confound the effect of education on mortality. Mathematical Population Studies, 16, 153–173.
Zheng, H., Yang, Y., & Land, K. C. (2011). Heterogeneity in the Strehler-Mildvan general theory of mortality and aging. Demography, 48, 267–290.
Acknowledgements
This research was supported by a graduate research fellowship from the National Science Foundation, a dissertation fellowship from the Ford Foundation, and core grants to the Center for Demography and Ecology (R24 HD047873) and Center for Demography of Health and Aging (P30 AG017266) at the University of Wisconsin–Madison. The author thanks, for helpful feedback, Felix Elwert, James Montgomery, Jenny Conrad, Michal Engelman, Duncan Gillespie, Jeffrey Grigg, Paul Hanselman, Anna Haskins, Michelle Niemann, Jenna Nobles, and Sarah Zureick-Brown, along with excellent reviewers and the Editor; and, for technical assistance, Russell Dimond, David Siegel, and the Social Science Computing Cooperative at the University of Wisconsin–Madison.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wrigley-Field, E. Mortality Deceleration and Mortality Selection: Three Unexpected Implications of a Simple Model. Demography 51, 51–71 (2014). https://doi.org/10.1007/s13524-013-0256-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13524-013-0256-7




 (slope) and highest
(slope) and highest  (intercept) values in the simulation universe. Excluding them does not appreciably change results.
(intercept) values in the simulation universe. Excluding them does not appreciably change results. = .002, and β = .103, has Gompertz intercept .009 and slope .081. It was chosen arbitrarily from among those cohorts exhibiting multiple relative decelerations whose aggregate parameters fell in a dense cluster of HMD cohorts, born in Sweden and Denmark in the mid-nineteenth century and in England, Wales, and Scotland in the late nineteenth century.
= .002, and β = .103, has Gompertz intercept .009 and slope .081. It was chosen arbitrarily from among those cohorts exhibiting multiple relative decelerations whose aggregate parameters fell in a dense cluster of HMD cohorts, born in Sweden and Denmark in the mid-nineteenth century and in England, Wales, and Scotland in the late nineteenth century.