Abstract
Background
Type-2 diabetes mellitus is a metabolic disorder characterised by hyperglycemia and insulin resistance. This study aims to explore the role and mechanism of DIRAS family GTPase 3 (DIRAS3) in mediating pancreatic β-cell death and insulin secretion.
Materials and Methods
A bioinformatic analysis of the GSE118230 and GSE150281 datasets was performed to screen differentially expressed genes. The pancreatic β-cell lines, INS-1 and MIN6, were treated with palmitic acid (PA) to mimic the cell models of type-2 diabetes mellitus. CCK-8 assay, 5-ethynyl-2’-deoxyuridine staining, flow cytometry, enzyme-linked immunoassay, immunofluorescence, qRT-PCR, and western immunoblotting were conducted to illustrate the role of DIRAS3 in the cell models.
Results
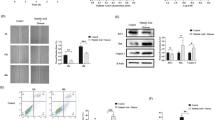
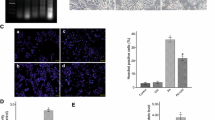
Unlike in normal controls, DIRAS3 was highly expressed in PA-treated pancreatic β-cells in a dose- and time-dependent manner. Moreover, the silencing of DIRAS3 in the INS-1 cells attenuated PA-induced cell loss by improving cell proliferation and inhibiting apoptosis and prevented the PA-induced impairment of insulin secretion. Consistently, the overexpression of DIRAS3 in the MIN6 cells accelerated PA-induced cell loss and impaired insulin secretion. A Gene Set Enrichment Analysis predicted that phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) was a downstream signalling pathway of DIRAS3, as the inhibitory effects of DIRAS3 on the activation of the PI3K/AKT signalling pathway were confirmed in the INS-1 and MIN6 cells. Moreover, the PI3K inhibitor, LY294002, effectively reversed the protective effects of DIRAS3 silencing on the INS-1 cells.
Conclusion
DIRAS3 was highly expressed in the cell models of type-2 diabetes mellitus, contributing to PA-induced cell death and impaired insulin secretion in pancreatic β-cells through the inhibition of the PI3K/AKT signalling pathway.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Libman I, Arslanian SA. Type II diabetes mellitus: no longer just adults. Pediatr Ann. 1999;28(9):589–93.
Ramachandran A, Snehalatha C, Ma RC. Diabetes in South-East Asia: an update. Diabetes Res Clin Pract. 2014;103(2):231–7.
Padhi S, Nayak AK, Behera A Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharm. = Biomedecine & pharmacotherapie (2020) 131:110708
Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27.
Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–96.
Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Dev (Cambridge, England). 2016;143(17):3050–60.
Matsuda S, Nakagawa Y, Tsuji A, Kitagishi Y, Nakanishi A, Murai T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Dis (Basel, Switzerland). 2018;6(2):28.
Nakano N, Matsuda S, Ichimura M, Minami A, Ogino M, Murai T, Kitagishi Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson’s disease (Review). Int J Mol Med. 2017;39(2):253–60.
Kumar M, Bansal N. Implications of Phosphoinositide 3-Kinase-Akt (PI3K-Akt) Pathway in the Pathogenesis of Alzheimer’s Disease. Mol Neurobiol. 2022;59(1):354–85.
Wang HJ, Ran HF, Yin Y, Xu XG, Jiang BX, Yu SQ, Chen YJ, Ren HJ, Feng S, Zhang JF, Chen Y, Xue Q, Xu XY. Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol Sin. 2021;43(7):1670–85.
Reddy NM, Potteti HR, Vegiraju S, Chen HJ, Tamatam CM, Reddy SP. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. Plos One. 2015;10(6): e0129676.
Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019;59:125–32.
Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Investig. 2004;114(7):963–8.
Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Investig. 2001;108(11):1631–8.
Liu Z, Hurst DR, Qu X, Lu LG, Wu CZ, Li YY, Li Y. Re-expression of DIRAS3 and p53 induces apoptosis and impaired autophagy in head and neck squamous cell carcinoma. Mil Med Res. 2020;7(1):48.
Li Y, Liu M, Zhang Y, Han C, You J, Yang J, Cao C, Jiao S. Effects of ARHI on breast cancer cell biological behavior regulated by microRNA-221. Tumour Biol The J Int Soc Oncodev Biol Med. 2013;34(6):3545–54.
Hu YQ, Si LJ, Ye ZS, Lin ZH, Zhou JP. Inhibitory effect of ARHI on pancreatic cancer cells and NF-κB activity. Mol Med Rep. 2013;7(4):1180–4.
Li X, Liu S, Fang X, He C, Hu X. The mechanisms of DIRAS family members in role of tumor suppressor. J Cell Physiol. 2019;234(5):5564–77.
Mao W, Peters HL, Sutton MN, Orozco AF, Pang L, Yang H, Lu Z, Bast RC Jr. The role of vascular endothelial growth factor, interleukin 8, and insulinlike growth factor in sustaining autophagic DIRAS3-induced dormant ovarian cancer xenografts. Cancer. 2019;125(8):1267–80.
Lu Z, Yang H, Sutton MN, Yang M, Clarke CH, Liao WS, Bast RC Jr. ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a-mediated induction of Rab7. Cell Death Differ. 2014;21(8):1275–89.
Allagnat F, Cunha D, Moore F, Vanderwinden JM, Eizirik DL, Cardozo AK. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to β-cell apoptosis. Cell Death Differ. 2011;18(2):328–37.
Hägglund MG, Hellsten SV, Bagchi S, Ljungdahl A, Nilsson VC, Winnergren S, Stephansson O, Rumaks J, Svirskis S, Klusa V, Schiöth HB, Fredriksson R. Characterization of the transporterB0AT3 (Slc6a17) in the rodent central nervous system. BMC Neurosci. 2013;14:54.
Peng Y, Jia J, Jiang Z, Huang D, Jiang Y, Li Y. Oncogenic DIRAS3 promotes malignant phenotypes of glioma by activating EGFR-AKT signaling. Biochem Biophys Res Commun. 2018;505(2):413–8.
Remedi MS, Emfinger C. Pancreatic β-cell identity in diabetes. Diab Obes Metab. 2016;181(1):110–6.
Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet (London, England). 2010;375(9733):2267–77.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–43.
Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, Man Y, Wang S, Yang J, Li J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285(39):29965–73.
Huang JS, Guo BB, Wang GH, Zeng LM, Hu YH, Wang T, Wang HY. DGAT1 inhibitors protect pancreatic β-cells from palmitic acid-induced apoptosis. Acta Pharmacol Sin. 2021;42(2):264–71.
Yin Y, Yong W, Yu J, Zhang X, Lin H, Zhu Y, Han X. Pdcd2l Promotes Palmitate-Induced Pancreatic Beta-Cell Apoptosis as a FoxO1 Target Gene. Plos One. 2016;11(11): e0166692.
Sutton MN, Yang H, Huang GY, Fu C, Pontikos M, Wang Y, Mao W, Pang L, Yang M, Liu J. RAS-related GTPases DIRAS1 and DIRAS2 induce autophagic cancer cell death and are required for autophagy in murine ovarian cancer cells. Autophagy. 2018;14(4):637–53.
Washington MN, Suh G, Orozco AF, Sutton MN, Yang H, Wang Y, Mao W, Millward S, Ornelas A, Atkinson N, Liao W, Bast RC Jr, Lu Z. ARHI (DIRAS3)-mediated autophagy-associated cell death enhances chemosensitivity to cisplatin in ovarian cancer cell lines and xenografts. Cell Death Dis. 2015;6(8): e1836.
Cui Z, Zeng Q, Guo Y, Liu S, Chen J. Integrated bioinformatic changes and analysis of retina with time in diabetic rats. PeerJ. 2018;6: e4762.
Zhuo C, Jiang R, Lin X, Shao M. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget. 2017;8(1):1429–37.
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu Z, Liu K, Sun H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol Res. 2018;130:466–80.
Bathina S, Gundala NKV, Rhenghachar P, Polavarapu S, Hari AD, Sadananda M, Das UN. Resolvin D1 Ameliorates Nicotinamide-streptozotocin-induced Type 2 Diabetes Mellitus by its Anti-inflammatory Action and Modulating PI3K/Akt/mTOR Pathway in the Brain. Arch Med Res. 2020;51(6):492–503.
Bildik G, Liang X, Sutton MN. DIRAS3: An Imprinted Tumor Suppressor Gene that Regulates RAS and PI3K-driven Cancer Growth, Motility, Autophagy, and Tumor Dormancy. Mol Cancer Ther. 2022;21(1):25–37.
Author information
Authors and Affiliations
Contributions
Ying Li designed the study; Ying Li, Shan Gao and Ying Yang performed the research; Gang Yin analyzed data; Gang Yin wrote the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
No conflicts and interests were involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. DIRAS3 is highly expressed in cell models of diabetes mellitus;.
2. DIRAS3 inhibits the proliferation of palmitic acid-treated pancreatic β‑cells;.
3. DIRAS3 induces apoptosis and insulin secretion impairment in pancreatic β‑cells;.
4. Silencing of DIRAS3 protects pancreatic β‑cells via regulating PI3K/AKT signalling.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Gao, S., Yang, Y. et al. Silencing of DIRAS3 improves the proliferation and insulin secretion of palmitic acid-treated pancreatic β‑cells through regulating PI3K/AKT signaling. Int J Diabetes Dev Ctries (2023). https://doi.org/10.1007/s13410-023-01240-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-023-01240-1