Abstract
Objectives
Maternal age at pregnancy is one of the most important risk factors for gestational diabetes mellitus (GDM); the particulars of the association vary by racial origin. Women less than 25 years old are considered to have low risk by the American Diabetes Association, but there are little data to support this among Chinese women. The aim of this study was to explore the relationship of maternal age and the incidence of GDM.
Methods
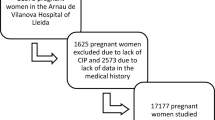
The data were drawn from a prenatal healthcare system and clinical record: 15,668 singleton pregnancies in women who had no pre-pregnancy diabetes and who became pregnant naturally. The relationships of age and GDM incidence were examined using χ2 and logistic regression models.
Results
The overall incidence of GDM was 22.72% (95% CI, 22.07–23.38). The incidence increased from 10.21% (95% CI, 8.18–12.14) in the age group of 18–22 years to 37.10% (95% CI, 33.71–40.49) in the age group of 36–49 years. The risk of GDM increased by an average of 8% for every 1 year of maternal age, and within each age group, the risk of GDM was 5% higher in primiparas than in pluriparas, in the range of age of 22 and 35 years.
Conclusions for practice
The incidence of GDM increased with maternal age. Women who got pregnant younger than 23 years had the lowest risk, followed by those who were younger than 30 years. The incidence GDM was especially high in women who were primiparas and were older than 30 years.


Similar content being viewed by others
References
World Health Organization. Diagnostic criteria and classification of hyperglycemia first detected in pregnancy. 2nd ed. Geneva: World Health Organization WHO/NMH/MND/13; 2013.
Herath H, Herath R, Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women-a community based retrospective cohort study. PLoS One. 2017;12(6):e0179647. https://doi.org/10.1371/journal.pone.0179647.
Chan JC, Cho NH, Tajima N, Shaw J. Diabetes in the Western Pacific Region--past, present and future. Diabetes Res Clin Pract. 2014;103(2):244–55. https://doi.org/10.1016/j.diabres.2013.11.012.
Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Ganesh Kumar S. Determinants of gestational diabetes mellitus: a case control study in a district tertiary care hospital in South India. Int J Diab Dev Countries. 2010;30(2):91–6. https://doi.org/10.4103/0973-3930.62599.
Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. https://doi.org/10.1007/s11892-015-0699-x.
Zhu WW, Yang HX, Wang C, Su RN, Feng H, Kapur A. High prevalence of gestational diabetes mellitus in Beijing: effect of maternal birth weight and other risk factors. Chin Med J. 2017;130(9):1019–25. https://doi.org/10.4103/0366-6999.204930.
Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, et al. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care. 2013;36(5):1209–14. https://doi.org/10.2337/dc12-0901.
Wu L, Han L, Zhan Y, Cui L, Chen W, Ma L, et al. Prevalence of gestational diabetes mellitus and associated risk factors in pregnant Chinese women: a cross-sectional study in Huangdao, Qingdao, China. Asia Pac J Clin Nutr. 2018;27(2):383–8. https://doi.org/10.6133/apjcn.032017.03.
Collier A, Abraham EC, Armstrong J, Godwin J, Monteath K, Lindsay R. Reported prevalence of gestational diabetes in Scotland: the relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J Diabetes Investig. 2017;8(2):161–7. https://doi.org/10.1111/jdi.12552.
Carolan M, Davey MA, Biro MA, Kealy M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery. 2012;28(6):778–83. https://doi.org/10.1016/j.midw.2011.08.014.
American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Supplement):S88–90.
Leng J, Shao P, Zhang C, Tian H, Zhang F, Zhang S, et al. Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: a prospective population-based study in Tianjin, China. PLoS One. 2015;10(3):e0121029. https://doi.org/10.1371/journal.pone.0121029.
Yang HX. Diagnostic criteria for gestational diabetes mellitus (WS 331-2011). Chin Med J. 2012;125(7):1212–3.
Liu X, Chen Y, Zhou Q, Shi H, Cheng WW. Utilization of International Association of Diabetes and Pregnancy Study Groups criteria vs. a two-step approach to screening for gestational diabetes mellitus in Chinese women with twin pregnancies. Diabet Med. 2015;32(3):367–73. https://doi.org/10.1111/dme.12636.
Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–13. https://doi.org/10.1111/1471-0528.14236.
Chernausek SD, Arslanian S, Caprio S, Copeland KC, El Ghormli L, Kelsey MM, et al. Relationship between parental diabetes and presentation of metabolic and glycemic function in youth with type 2 diabetes: baseline findings from the TODAY trial. Diabetes Care. 2016;39(1):110–7. https://doi.org/10.2337/dc15-1557.
Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–85. https://doi.org/10.1016/j.diabres.2013.11.003.
Liao S, Mei J, Song W, Liu Y, Tan YD, Chi S, et al. The impact of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) fasting glucose diagnostic criterion on the prevalence and outcomes of gestational diabetes mellitus in Han Chinese women. Diabet Med. 2014;31(3):341–51. https://doi.org/10.1111/dme.12349.
Karcaaltincaba D, Calis P, Ocal N, Ozek A, Altug Inan M, Bayram M. Prevalence of gestational diabetes mellitus evaluated by universal screening with a 75-g, 2-hour oral glucose tolerance test and IADPSG criteria. Int J Gynaecol Obstet. 2017;138(2):148–51. https://doi.org/10.1002/ijgo.12205.
Laine MK, Kautiainen H, Gissler M, Raina M, Aahos I, Jarvinen K, et al. Gestational diabetes in primiparous women-impact of age and adiposity: a register-based cohort study. Acta Obstet Gynecol Scand. 2018;97(2):187–94. https://doi.org/10.1111/aogs.13271.
Baeyens L, Hindi S, Sorenson RL, German MS. beta-Cell adaptation in pregnancy. Diabetes Obes Metab. 2016;18(Suppl 1):63–70. https://doi.org/10.1111/dom.12716.
Banerjee RR. Piecing together the puzzle of pancreatic islet adaptation in pregnancy. Ann N Y Acad Sci. 2018;1411(1):120–39. https://doi.org/10.1111/nyas.13552.
Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95(10):E234–9. https://doi.org/10.1210/jc.2010-0932.
Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–76. https://doi.org/10.1007/s00125-010-1809-6.
Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31(3):539–43. https://doi.org/10.2337/dc07-1443.
Sweeting AN, Wong J, Appelblom H, Ross GP, Kouru H, Williams PF, et al. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther. 2019;45(2):76–84. https://doi.org/10.1159/000486853.
Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018;145:130–7. https://doi.org/10.1016/j.diabres.2018.05.001.
Feleke BE. Determinants of gestational diabetes mellitus: a case-control study. J Matern Fetal Neonatal Med. 2018;31(19):2584–9. https://doi.org/10.1080/14767058.2017.1347923.
Hao M, Lin L. Fasting plasma glucose and body mass index during the first trimester of pregnancy as predictors of gestational diabetes mellitus in a Chinese.
Funding
The study received funding from the National Key Research and Development Program, Ministry of Science and Technology of the People’s Republic of China (Grant Nos. 2016YFC1000500 and 2016YFC1000501). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Ministry of Science and Technology. The funding agents of the study played no role in its design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The researchers are independent from the funding agents, and all authors, external and internal, had full access to all study data (including statistical reports and tables) and can take responsibility for the integrity of the data and the accuracy of the analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The data we used for research was from regular healthcare, for which we did not need the informed consent. The study was approved by the Institutional Review Board of Peking University (IRB00001052-18010).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yating Han and Mingkun Tong have equal authorship.
Significance
Previous studies have found that maternal age at pregnancy is one of the most important risk factors for GDM. Women less than 25 years old are considered to have low risk by the American Diabetes Association, but there are little data to support this among Chinese women. We explored the association between maternal age and risk for GDM in China and found: the incidence of GDM increased with maternal age at pregnancy, and the risk for GDM increased by an average of 8% for every year of maternal age between 23 and 36 years old; for Chinese women, reproduction at a younger age may reduce the incidence of GDM; for prevention GDM in China, primiparity should be encouraged at younger ages than 23 years old or at least not older than 30 years old.
Rights and permissions
About this article
Cite this article
Han, Y., Tong, M., Jin, L. et al. Maternal age at pregnancy and risk for gestational diabetes mellitus among Chinese women with singleton pregnancies. Int J Diabetes Dev Ctries 41, 114–120 (2021). https://doi.org/10.1007/s13410-020-00859-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-020-00859-8