Abstract
Objective
To study risk factors for tuberculosis (TB) patients with diabetes mellitus (DM) living in Western China and analyze the baseline characteristics and clinical data of those patients for developing an effective screening strategy.
Methods
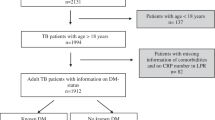
We enrolled 3548 TB patients who were admitted to our hospital from 2014 to 2018. The baseline characteristics and clinical data of TB patients with and without DM were compared. Besides, risk factors were presented, and their effects on TB patients with and without DM were analyzed.
Results
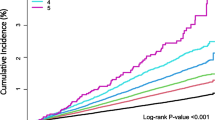
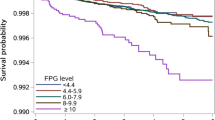
The prevalence of DM among TB patients was 7.7%, which increased with elevation of the patients’ age, and 63.1% of TB patients with DM had hemoglobin A1c (HbA1c) ≥ 7.0%. The prevalence of DM in the Han patients with TB was the highest (8.8%), which was roughly three times higher than that in the Tibetan patients with TB (3.0%). In the multivariate logistic regression analysis, elevated values of the patients’ age (odds ratio (OR), 1.047 (1.033–1.062, p < 0.01), blood pressure (OR, 1.735 (1.101–2.734), p = 0.04), proportion of cavity in pulmonary TB (PTB) (OR, 2.167 (1.272–3.656), p = 0.004), fasting blood glucose (OR, 2.248 (1.997–2.555), p < 0.001), erythrocyte sedimentation rate (ESR) (OR, 1.007 (1.001–1.012), p = 0.027), and proportion of patients with PTB (OR, 2.426 (1.425–4.104), p < 0.001) were significantly associated with increased prevalence of DM in TB patients. For evaluation of the model, the receiver operating characteristic (ROC) curve was plotted, in which the area under the curve (AUC) value of 0.924 was obtained for an optimal cutoff value of 0.052. The re-sampling method was utilized to verify the regression model, and the mean squared error (MSE) was 0.00026.
Conclusions
The prevalence of DM in TB patients is high and is associated with severe clinical symptoms. Therefore, early screening of DM for TB patients is highly recommended.


Similar content being viewed by others
References
WHO. Global report on diabetes. Geneva: World Health Organization; 2016. p. 2016.
Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis. 2008;8:8.
Narayan KM, Gregg EW, Fagot-Campagna A, Engelgau MM, Vinicor F. Diabetes--a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract. 2000;50(Suppl 2):S77–84.
Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–46.
Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Tropical medicine & international health : TM & IH. 2010;15(11):1289–99.
Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152.
Kuo MC, Lin SH, Lin CH, Mao IC, Chang SJ, Hsieh MC. Type 2 diabetes: an independent risk factor for tuberculosis: a nationwide population-based study. PLoS One. 2013;8(11):e78924.
Pan SC, Ku CC, Kao D, Ezzati M, Fang CT, Lin HH. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. Lancet Diabetes Endocrinol. 2015;3(5):323–30.
Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57(2):233–45.
Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2(9):730–9.
National Technical Steering Group of the Epidemiological Sampling Survey for Tuberculosis, Implementing Office of the Epidemiological Sampling Survey for Tuberculosis. The prevalence of pulmonary tuberculosis in a national survey across China in 2010. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35(9):665–8.
Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104(1):63–72.
Feng Y, Yang Y, Ma X, et al. Prevalence of diabetes among Han, Manchu and Korean ethnicities in the Mudanjiang area of China: a cross-sectional survey. BMC Public Health. 2012;12:23.
Demir M, Serin E, Gokturk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur J Gastroenterol Hepatol. 2008;20(7):668–73.
Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural south India. Journal of infection and public health. 2011;4(3):140–4.
Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68(3):214–20.
Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLoS One. 2016;11(1):e0146876.
Duo L, Ying B, Song X, Lu X, Ye Y, Fan H, et al. Molecular profile of drug resistance in tuberculous meningitis from southwest china. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1067–73.
Wu Z, Guo J, Huang Y, Cai E, Zhang X, Pan Q, et al. Diabetes mellitus in patients with pulmonary tuberculosis in an aging population in Shanghai, China: prevalence, clinical characteristics and outcomes. J Diabetes Complicat. 2016;30(2):237–41.
Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: a community based cohort study. PLoS One. 2013;8(12):e82660.
Li L, Lin Y, Mi F, Tan S, Liang B, Guo C, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Tropical medicine & international health : TM & IH. 2012;17(10):1294–301.
Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184(11):6275–82.
Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208(5):739–48.
Tian PW, Wang Y, Shen YC, Chen L, Wan C, Liao ZL, et al. Different risk factors of recurrent pulmonary tuberculosis between Tibetan and Han populations in Southwest China. Eur Rev Med Pharmacol Sci. 2014;18(10):1482–6.
Ren G, You J, Gong X, Zhang X, Zhao L, Wei X, et al. SP110 and PMP22 polymorphisms are associated with tuberculosis risk in a Chinese-Tibetan population. Oncotarget. 2016;7(40):66100–8.
Song X, Li S, QuCuo M, Zhou M, Zhou Y, Hu X, et al. Association between SNPs in microRNA-machinery genes and tuberculosis susceptibility in Chinese Tibetan population. Mol Biol Rep. 2013;40(10):6027–33.
Chiang CY, Lee JJ, Chien ST, Enarson DA, Chang YC, Chen YT, et al. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PLoS One. 2014;9(4):e93397.
Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167(12):1486–94.
Magee MJ, Kempker RR, Kipiani M, Gandhi NR, Darchia L, Tukvadze N, et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(6):685–92.
Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLoS One. 2016;11(1):e0147621.
Almeida-Junior JL, Gil-Santana L, Oliveira CA, et al. Glucose metabolism disorder is associated with pulmonary tuberculosis in individuals with respiratory symptoms from Brazil. PLoS One. 2016;11(4):e0153590.
Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7(7):e41367.
Nair A, Guleria R, Kandasamy D, Sharma R, Tandon N, Singh UB, et al. Prevalence of pulmonary tuberculosis in young adult patients with type 1 diabetes mellitus in India. Multidiscip Respir Med. 2016;11:22.
Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144(2):171–85.
Cabrera SM, Henschel AM, Hessner MJ. Innate inflammation in type 1 diabetes. Translational research : the journal of laboratory and clinical medicine. 2016;167(1):214–27.
Cejkova P, Nemeckova I, Broz J, Cerna M. TLR2 and TLR4 expression on CD14(++) and CD14(+) monocyte subtypes in adult-onset autoimmune diabetes. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2016;160(1):76–83.
Acknowledgment
We highly appreciate participation of all subjects in this study and assistance of clinicians who contributed to blood sampling and data collection.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 81902142, 81672095, and 81501800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study received approval from the Ethics Committee of West China Hospital, Sichuan University (Approval No. 198 (2014)).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1a
Lifestyle and TB-related clinical symptoms of the different ethnics. a. The percentage of patients with smoking history was around 30%; the lowest rate was 16.5% for is Tibetan patients. b. The percentage of patients with alcohol intake was about 20%; c. Except for the Han patients, the percentage of rural residents in minority ethnic groups was higher than that of urban dwellers; d. & g. The percentages of cough and anorexia were the highest in the Tibetan patients; e. The Tibetan patients had the highest proportion of weight loss; f. The Yi patients had the highest proportion of night sweats; h. The proportion of the Han patients with fever was the highest and that of Qiang patients was the lowest. (PNG 206 kb)
Fig. S1b
(PNG 207 kb)
Fig. S1c
(PNG 208 kb)
Fig. S1d
(PNG 209 kb)
Fig. S1e
(PNG 220 kb)
Fig. S1f
(PNG 213 kb)
Fig. S1g
(PNG 213 kb)
Fig. S1h
(PNG 206 kb)
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
He, H., Zhang, M., Song, X. et al. The risk factors for tuberculosis patients with diabetes mellitus living in Western China: a retrospective study conducted from 2014 to 2018. Int J Diabetes Dev Ctries 40, 538–546 (2020). https://doi.org/10.1007/s13410-020-00834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-020-00834-3