Abstract
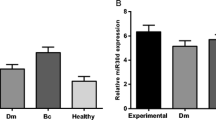
Type 2 diabetes mellitus (T2DM) increases the incidence of post-menopausal breast cancer (PMBC). This study is intended to determine whether microRNA-103/107 (miR-103/107) should be regarded as a potential molecular link between T2DM and PMBC. Samples of serum from 90 patients with T2DM and/or PMBC were collected. Samples of serum from 20 non-diabetic post-menopausal women were used as the control. The body mass index (BMI) of patients with T2DM and PMBC was lower than the BMI of patients with only T2DM or PMBC (p < 0.05). The expression of miR-103/107 was higher in the serum of T2DM patients compared with that in control samples (2.80 ± 0.46/36.29 ± 3.41 vs 0.88 ± 0.25/8.59 ± 1.91, p < 0.05). The expression of miR-103/107 in the serum of PMBC patients was higher than that in T2DM patients (5.06 ± 0.92/49.59 ± 6.99 vs 2.80 ± 0.46/36.29 ± 3.41, p < 0.05) but lower than that in patients diagnosed with both T2DM and PMBC (7.67 ± 0.87/63.24 ± 8.58, p < 0.05). miR-103/107 was positively correlated with the homeostasis model assessment-insulin resistance (HOMA-IR) index (r = 0.71, 0.685, p < 0.01). The expression of miR-103/107 was an independent factor of the HOMA-IR index (β = 0.638, 0.073, p = 0.02, 0.01). There were higher levels of estradiol (E2) in patients with T2DM and/or PMBC than that in the control group. High expression of miR-103/107 results in insulin resistance and is associated with overweight or obese patients with T2DM and PMBC through elevated levels of E2. miR-103/107 may be a potential molecular link between T2DM and PMBC.
Similar content being viewed by others
References
Gouveri E, Papanas N, Maltezos E. The female breast and diabetes. Breast. 2011;20(3):205–11.
Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608–17.
Wang XL, Jia CX, Liu LY, Zhang Q, Li YY, Li L. Obesity, diabetes mellitus, and the risk of female breast cancer in eastern China. World J Surg Oncol. 2013;11:71.
La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B. Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle. Oncologist. 2011;16(6):726–9.
Oh YS, Cho KA, Ryu SJ, Khil LY, Jun HS, Yoon JW, et al. Regulation of insulin response in skeletal muscle cell by caveolin status. J Cell Biochem. 2006;99(3):747–58.
Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, et al. A microRNA targeting dicer for metastasis control. Cell. 2010;141(7):1195–207.
Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649–53.
Mercier I, Lisanti MP. Caveolin-1 and breast cancer: a new clinical perspective. Adv Exp Med Biol. 2012;729:83–94.
Brewster BL, Rossiello F, French JD, Edwards SL, Wong M, Wronski A, et al. Identification of fifteen novel germline variants in the BRCA1 3′UTR reveals a variant in a breast cancer case that introduces a functional miR-103 target site. Hum Mutat. 2012;33(12):1665–75.
Kleivi Sahlberg K, Bottai G, Naume B, Burwinkel B, Calin GA, Borresen-Dale AL, et al. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res. 2015;21(5):1207–14.
Wu Q, Wang C, Lu Z, Guo L, Ge Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clin Chim Acta: Int J Clin Chem. 2012;413(13–14):1058–65.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med: J Br Diabet Assoc. 1998;15(7):539–53.
St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27(9):2222–8.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Levi J, Gray SL, Speck M, Huynh FK, Babich SL, Gibson WT, et al. Acute disruption of leptin signaling in vivo leads to increased insulin levels and insulin resistance. Endocrinology. 2011;152(9):3385–95.
Lin L, Pang W, Chen K, Wang F, Gengler J, Sun Y, et al. Adipocyte expression of PU.1 transcription factor causes insulin resistance through upregulation of inflammatory cytokine gene expression and ROS production. Am J Physiol Endocrinol Metab. 2012;302(12):E1550–9.
Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2015. 99 (Part A): 61–66.
Vicennati V, Garelli S, Rinaldi E, Rosetti S, Zavatta G, Pagotto U, et al. Obesity-related proliferative diseases: the interaction between adipose tissue and estrogens in post-menopausal women. Horm Mol Biol Clin Investig. 2015;21(1):75–87.
Madeddu C, Gramignano G, Floris C, Murenu G, Sollai G, Maccio A. Role of inflammation and oxidative stress in post-menopausal oestrogen-dependent breast cancer. J Cell Mol Med. 2014;18(12):2519–29.
Kim JY, Han W, Moon HG, Ahn SK, Kim J, Lee JW, et al. Prognostic effect of preoperative serum estradiol level in postmenopausal breast cancer. BMC Cancer. 2013;13:503.
Mezza T, Muscogiuri G, Sorice GP, Clemente G, Hu J, Pontecorvi A, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63(3):994–1007.
Sieri S, Muti P, Claudia A, Berrino F, Pala V, Grioni S, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J Cancer. 2012;130(4):921–9.
Weichhaus M, Broom J, Wahle K, Bermano G. A novel role for insulin resistance in the connection between obesity and postmenopausal breast cancer. Int J Oncol. 2012;41(2):745–52.
Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol: Off J Int Soc Gynecol Endocrinol. 2013;29(3):263–7.
Acknowledgments
We thank Ye Wang (Shandong Eye Institute, No. 5 Yan’erdao Rd. Qingdao, 266071, China) for the valuable assistance.
M.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the editorial assistance of the American Journal Experts (Durham, NC, USA) for help with preparing the manuscript.
Conflicts of interest
No potential conflicts of interest relevant to this article are reported.
Author contributions
Q.X., Y.S., and Y.L. participated in the conception and design of the study and the critical revision of the manuscript for important intellectual content. Q.X., Y.S., F.Z., S.G., and M.Y. performed the data collection and analysis. Q.X. interpreted the data and produced the draft of the manuscript. M.Y. obtained funding for the study. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Q., Shang, Y., Li, Y. et al. MicroRNAs 103 and 107 link type 2 diabetes and post-menopausal breast cancer. Int J Diabetes Dev Ctries 36, 40–44 (2016). https://doi.org/10.1007/s13410-015-0412-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-015-0412-2